1
JEE Advanced 2021 Paper 2 Online
Numerical
+4
-0

One mole of an ideal gas at 900 K, undergoes two reversible processes, I followed by II, as shown below. If the work done by the gas in the two processes are same, the value of $$\ln {{{V_3}} \over {{V_2}}}$$ is _________.
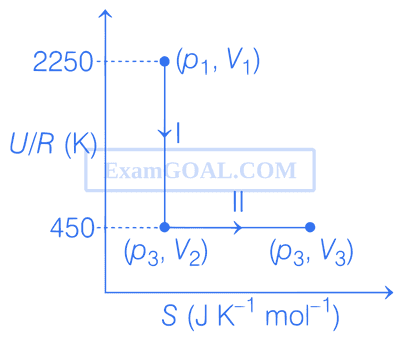
(U : internal energy, S : entropy, p : pressure, V : volume, R : gas constant)
(Given : molar heat capacity at constant volume, CV,m of the gas is $${5 \over 2}$$R)

(U : internal energy, S : entropy, p : pressure, V : volume, R : gas constant)
(Given : molar heat capacity at constant volume, CV,m of the gas is $${5 \over 2}$$R)
Your input ____
2
JEE Advanced 2021 Paper 2 Online
Numerical
+4
-0

Consider a helium (He) atom that absorbs a photon of wavelength 330 nm. The change in the velocity (in cm s$$-$$1) of He atom after the photon absorption is __________.
(Assume : Momentum is conserved when photon is absorbed.
Use : Planck constant = 6.6 $$\times$$ 10$$-$$34 J s, Avogadro number = 6 $$\times$$ 1023 mol$$-$$1, Molar mass of He = 4 g mol$$-$$1)
(Assume : Momentum is conserved when photon is absorbed.
Use : Planck constant = 6.6 $$\times$$ 10$$-$$34 J s, Avogadro number = 6 $$\times$$ 1023 mol$$-$$1, Molar mass of He = 4 g mol$$-$$1)
Your input ____
3
JEE Advanced 2021 Paper 2 Online
Numerical
+4
-0

Ozonolysis of ClO2 produces an oxide of chlorine. The average oxidation state of chlorine in this oxide is __________.
Your input ____
4
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

Let
$${S_1} = \left\{ {(i,j,k):i,j,k \in \{ 1,2,....,10\} } \right\}$$,
$${S_2} = \left\{ {(i,j):1 \le i < j + 2 \le 10,i,j \in \{ 1,2,...,10\} } \right\}$$,
$${S_3} = \left\{ {(i,j,k,l):1 \le i < j < k < l,i,j,k,l \in \{ 1,2,...,10\} } \right\}$$ and
$${S_4} = \{ (i,j,k,l):i,j,k$$ and $$l$$ are distinct elements in {1, 2, ...., 10}.
If the total number of elements in the set Sr is nr, r = 1, 2, 3, 4, then which of the following statements is(are) TRUE?
$${S_1} = \left\{ {(i,j,k):i,j,k \in \{ 1,2,....,10\} } \right\}$$,
$${S_2} = \left\{ {(i,j):1 \le i < j + 2 \le 10,i,j \in \{ 1,2,...,10\} } \right\}$$,
$${S_3} = \left\{ {(i,j,k,l):1 \le i < j < k < l,i,j,k,l \in \{ 1,2,...,10\} } \right\}$$ and
$${S_4} = \{ (i,j,k,l):i,j,k$$ and $$l$$ are distinct elements in {1, 2, ...., 10}.
If the total number of elements in the set Sr is nr, r = 1, 2, 3, 4, then which of the following statements is(are) TRUE?
Paper analysis
Total Questions
Chemistry
19
Mathematics
19
Physics
19
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004
IIT-JEE 2004 Screening
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001
IIT-JEE 2001 Screening
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998
IIT-JEE 1998 Screening
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978