1
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

Correct option(s) for the following sequence of reactions is(are)
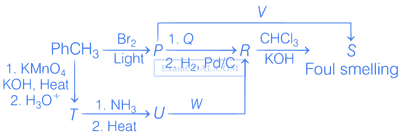

2
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

For the following reaction,
$$2X + Y\buildrel k \over \longrightarrow P$$ the rate of reaction is $${{d[P]} \over {dt}} = k[X]$$. Two moles of X are mixed with one mole of Y to make 1.0 L of solution. At 50 s, 0.5 mole of Y is left in the reaction mixture. The correct statement(s) about the reaction is(are)
(Use : ln 2 = 0.693)
$$2X + Y\buildrel k \over \longrightarrow P$$ the rate of reaction is $${{d[P]} \over {dt}} = k[X]$$. Two moles of X are mixed with one mole of Y to make 1.0 L of solution. At 50 s, 0.5 mole of Y is left in the reaction mixture. The correct statement(s) about the reaction is(are)
(Use : ln 2 = 0.693)
3
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

Some standard electrode potentials at 298 K are given below :
Pb2+ /Pb = $$- $$0.13 V
Ni2+ /Ni = $$-$$ 0.24 V
Cd2+ /Cd = $$-$$ 0.40 V
Fe2+ /Fe = $$-$$ 0.44 V
To a solution containing 0.001 M of X2+ and 0.1 M of Y2+, the metal rods X and Y are inserted (at 298 K) and connected by a conducting wire. This resulted in dissolution of X. The correct combination(s) of X and Y, respectively, is(are)
(Given : Gas constant, R = 8.314 J K$$-$$ mol$$-$$1, Faraday constant, F = 96500 C mol$$-$$1)
Pb2+ /Pb = $$- $$0.13 V
Ni2+ /Ni = $$-$$ 0.24 V
Cd2+ /Cd = $$-$$ 0.40 V
Fe2+ /Fe = $$-$$ 0.44 V
To a solution containing 0.001 M of X2+ and 0.1 M of Y2+, the metal rods X and Y are inserted (at 298 K) and connected by a conducting wire. This resulted in dissolution of X. The correct combination(s) of X and Y, respectively, is(are)
(Given : Gas constant, R = 8.314 J K$$-$$ mol$$-$$1, Faraday constant, F = 96500 C mol$$-$$1)
4
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

The pair(s) of complexes wherein both exhibit tetrahedral geometry is(are)
(Note : py = pyridine)
Given : Atomic numbers of Fe, Co, Ni and Cu are 26, 27, 28 and 29, respectively)
(Note : py = pyridine)
Given : Atomic numbers of Fe, Co, Ni and Cu are 26, 27, 28 and 29, respectively)
Paper analysis
Total Questions
Chemistry
19
Mathematics
19
Physics
19
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004
IIT-JEE 2004 Screening
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001
IIT-JEE 2001 Screening
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998
IIT-JEE 1998 Screening
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978