1
JEE Advanced 2021 Paper 2 Online
MCQ (Single Correct Answer)
+3
-1

The amount of energy required to break a bond is same as the amount of energy released when the same bond is formed. In gaseous state, the energy required for homolytic cleavage of a bond is called Bond Dissociation Energy (BDE) or Bond Strength. BDE is affected by s-character of the bond and the stability of the radicals formed. Shorter bonds are typically stronger bonds. BDEs for some bonds are given below :

Cl $$-$$ Cl (g) $$\to$$ Cl$$_{(g)}^ \bullet $$ + Cl$$_{(g)}^ \bullet $$ $$\Delta$$H$$^\circ$$ = 58 kcal mol$$-$$1
H3C $$-$$ Cl (g) $$\to$$ H3C$$_{(g)}^ \bullet $$ + Cl$$_{(g)}^ \bullet $$ $$\Delta$$H$$^\circ$$ = 85 kcal mol$$-$$1
H $$-$$ Cl (g) $$\to$$ H$$_{(g)}^ \bullet $$ + Cl$$_{(g)}^ \bullet $$ $$\Delta$$H$$^\circ$$ = 103 kcal mol$$-$$1

Cl $$-$$ Cl (g) $$\to$$ Cl$$_{(g)}^ \bullet $$ + Cl$$_{(g)}^ \bullet $$ $$\Delta$$H$$^\circ$$ = 58 kcal mol$$-$$1
H3C $$-$$ Cl (g) $$\to$$ H3C$$_{(g)}^ \bullet $$ + Cl$$_{(g)}^ \bullet $$ $$\Delta$$H$$^\circ$$ = 85 kcal mol$$-$$1
H $$-$$ Cl (g) $$\to$$ H$$_{(g)}^ \bullet $$ + Cl$$_{(g)}^ \bullet $$ $$\Delta$$H$$^\circ$$ = 103 kcal mol$$-$$1
For the following reaction
CH4(g) + Cl2(g) $$\buildrel {light} \over \longrightarrow $$ CH3Cl(g) + HCl (g)
the correct statement is
CH4(g) + Cl2(g) $$\buildrel {light} \over \longrightarrow $$ CH3Cl(g) + HCl (g)
the correct statement is
2
JEE Advanced 2021 Paper 2 Online
MCQ (Single Correct Answer)
+3
-1

The reaction of K3[Fe(CN)6] with freshly prepared FeSO4 solution produces a dark blue precipitate called Turnbull's blue. Reaction of K4[Fe(CN)6] with the FeSO4 solution in complete absence of air produces a white precipitate X, which turns blue in air. Mixing the FeSO4 solution with NaNO3, followed by a slow addition of concentrated H2SO4 through the side of the test tube produces a brown ring.
Precipitate X is
3
JEE Advanced 2021 Paper 2 Online
MCQ (Single Correct Answer)
+3
-1

The reaction of K3[Fe(CN)6] with freshly prepared FeSO4 solution produces a dark blue precipitate called Turnbull's blue. Reaction of K4[Fe(CN)6] with the FeSO4 solution in complete absence of air produces a white precipitate X, which turns blue in air. Mixing the FeSO4 solution with NaNO3, followed by a slow addition of concentrated H2SO4 through the side of the test tube produces a brown ring.
Among the following, the brown ring is due to the formation of
4
JEE Advanced 2021 Paper 2 Online
Numerical
+4
-0

One mole of an ideal gas at 900 K, undergoes two reversible processes, I followed by II, as shown below. If the work done by the gas in the two processes are same, the value of $$\ln {{{V_3}} \over {{V_2}}}$$ is _________.
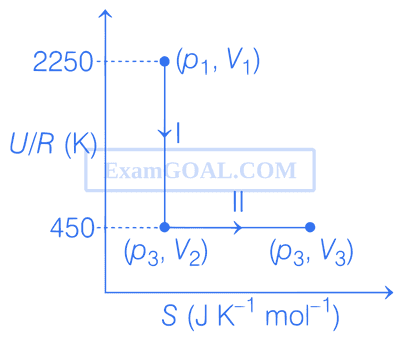
(U : internal energy, S : entropy, p : pressure, V : volume, R : gas constant)
(Given : molar heat capacity at constant volume, CV,m of the gas is $${5 \over 2}$$R)

(U : internal energy, S : entropy, p : pressure, V : volume, R : gas constant)
(Given : molar heat capacity at constant volume, CV,m of the gas is $${5 \over 2}$$R)
Your input ____
Paper analysis
Total Questions
Chemistry
19
Mathematics
19
Physics
19
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004
IIT-JEE 2004 Screening
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001
IIT-JEE 2001 Screening
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998
IIT-JEE 1998 Screening
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978