1
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

The reaction sequence(s) that would lead to o-xylene as the major product is(are)
2
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

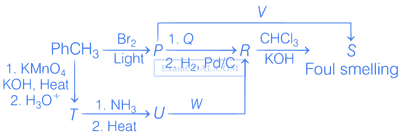
Correct option(s) for the following sequence of reactions is(are)

3
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

For the following reaction,
$$2X + Y\buildrel k \over \longrightarrow P$$ the rate of reaction is $${{d[P]} \over {dt}} = k[X]$$. Two moles of X are mixed with one mole of Y to make 1.0 L of solution. At 50 s, 0.5 mole of Y is left in the reaction mixture. The correct statement(s) about the reaction is(are)
(Use : ln 2 = 0.693)
$$2X + Y\buildrel k \over \longrightarrow P$$ the rate of reaction is $${{d[P]} \over {dt}} = k[X]$$. Two moles of X are mixed with one mole of Y to make 1.0 L of solution. At 50 s, 0.5 mole of Y is left in the reaction mixture. The correct statement(s) about the reaction is(are)
(Use : ln 2 = 0.693)
4
JEE Advanced 2021 Paper 2 Online
MCQ (More than One Correct Answer)
+4
-2

Some standard electrode potentials at 298 K are given below :
Pb2+ /Pb = $$- $$0.13 V
Ni2+ /Ni = $$-$$ 0.24 V
Cd2+ /Cd = $$-$$ 0.40 V
Fe2+ /Fe = $$-$$ 0.44 V
To a solution containing 0.001 M of X2+ and 0.1 M of Y2+, the metal rods X and Y are inserted (at 298 K) and connected by a conducting wire. This resulted in dissolution of X. The correct combination(s) of X and Y, respectively, is(are)
(Given : Gas constant, R = 8.314 J K$$-$$ mol$$-$$1, Faraday constant, F = 96500 C mol$$-$$1)
Pb2+ /Pb = $$- $$0.13 V
Ni2+ /Ni = $$-$$ 0.24 V
Cd2+ /Cd = $$-$$ 0.40 V
Fe2+ /Fe = $$-$$ 0.44 V
To a solution containing 0.001 M of X2+ and 0.1 M of Y2+, the metal rods X and Y are inserted (at 298 K) and connected by a conducting wire. This resulted in dissolution of X. The correct combination(s) of X and Y, respectively, is(are)
(Given : Gas constant, R = 8.314 J K$$-$$ mol$$-$$1, Faraday constant, F = 96500 C mol$$-$$1)
Paper analysis
Total Questions
Chemistry
19
Mathematics
19
Physics
19
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978



