
$2 \mathrm{~mol} \,\mathrm{of}\, \mathrm{Hg}(\mathrm{g})$ is combusted in a fixed volume bomb calorimeter with excess of $\mathrm{O}_{2}$ at $298 \mathrm{~K}$ and 1 atm into $\mathrm{HgO}(s)$. During the reaction, temperature increases from $298.0 \mathrm{~K}$ to $312.8 \mathrm{~K}$. If heat capacity of the bomb calorimeter and enthalpy of formation of $\mathrm{Hg}(g)$ are $20.00 \mathrm{~kJ} \mathrm{~K}^{-1}$ and $61.32 \mathrm{~kJ}$ $\mathrm{mol}^{-1}$ at $298 \mathrm{~K}$, respectively, the calculated standard molar enthalpy of formation of $\mathrm{HgO}(s)$ at 298 $\mathrm{K}$ is $\mathrm{X}\, \mathrm{kJ}\, \mathrm{mol}^{-1}$. The value of $|\mathrm{X}|$ is _________ .
[Given: Gas constant $\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ ]

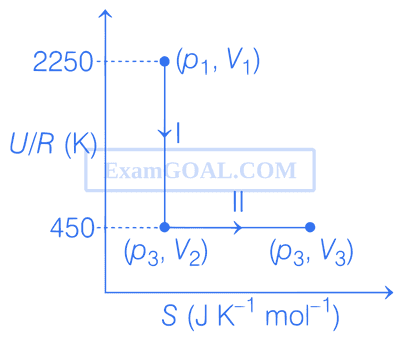
(U : internal energy, S : entropy, p : pressure, V : volume, R : gas constant)
(Given : molar heat capacity at constant volume, CV,m of the gas is $${5 \over 2}$$R)

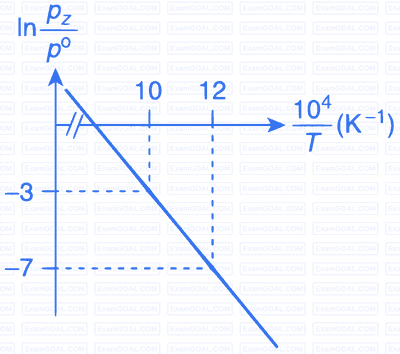
(Given, $${{d(\ln K)} \over {d\left( {{1 \over T}} \right)}} = - {{\Delta {H^\theta }} \over R}$$, where the equilibrium
constant, $$K = {{pz} \over {{p^\theta }}}$$ and the gas constant, R = 8.314 J K$$-$$1 mol$$-$$1)


(Given, $${{d(\ln K)} \over {d\left( {{1 \over T}} \right)}} = - {{\Delta {H^\theta }} \over R}$$, where the equilibrium
constant, $$K = {{pz} \over {{p^\theta }}}$$ and the gas constant, R = 8.314 J K$$-$$1 mol$$-$$1)