
An electrochemical cell is fueled by the combustion of butane at 1 bar and 298 K . Its cell potential is $\frac{\boldsymbol{X}}{F} \times 10^3$ volts, where $F$ is the Faraday constant. The value of $\boldsymbol{X}$ is _____________.
Use: Standard Gibbs energies of formation at 298 K are: $\Delta_f G_{\mathrm{CO}_2}^o=-394 \mathrm{~kJ} \mathrm{~mol}^{-1} ; \Delta_f G_{\text {water }}^o=$ $-237 \mathrm{~kJ} \mathrm{~mol}^{-1} ; \Delta_f G_{\text {butane }}^o=-18 \mathrm{~kJ} \mathrm{~mol}^{-1}$

In an electrochemical cell, dichromate ions in aqueous acidic medium are reduced to Cr3+. The current (in amperes) that flows through the cell for 48.25 minutes to produce 1 mole of Cr3+ is ______.
Use: 1 Faraday = 96500 C mol−1

Given:
| Ion | $\mathrm{Z}^{\mathrm{n}+}$ | $\mathrm{U}^{\mathrm{p}+}$ | $\mathrm{V}^{\mathrm{n}+}$ | $\mathrm{X}^{\mathrm{m}-}$ | $\mathrm{Y}^{\mathrm{m}-}$ |
|---|---|---|---|---|---|
| $\lambda^{0}\left(\mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}\right)$ | $50.0$ | $25.0$ | $100.0$ | $80.0$ | $100.0$ |
$\lambda^{0}$ is the limiting molar conductivity of ions
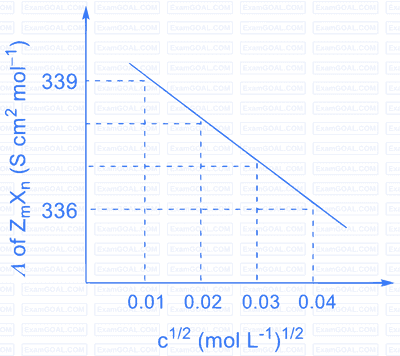
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.

The reduction potential $\left(E^{0}\right.$, in $\left.\mathrm{V}\right)$ of $\mathrm{MnO}_{4}^{-}(\mathrm{aq}) / \mathrm{Mn}(\mathrm{s})$ is __________.
[Given: $E_{\left(\mathrm{MnO}_{4}^{-}(\mathrm{aq}) / \mathrm{MnO}_{2}(\mathrm{~s})\right)}^{0}=1.68 \mathrm{~V} ; E_{\left(\mathrm{MnO}_{2}(\mathrm{~s}) / \mathrm{Mn}^{2+}(\mathrm{aq})\right)}^{0}=1.21 \mathrm{~V} ; E_{\left(\mathrm{Mn}^{2+}(\mathrm{aq}) / \mathrm{Mn}(\mathrm{s})\right)}^{0}=-1.03 \mathrm{~V}$ ]