1
JEE Advanced 2023 Paper 1 Online
Numerical
+4
-0

The plot of $\log k_f$ versus $1 / T$ for a reversible reaction $\mathrm{A}(\mathrm{g}) \rightleftharpoons \mathrm{P}(\mathrm{g})$ is shown.
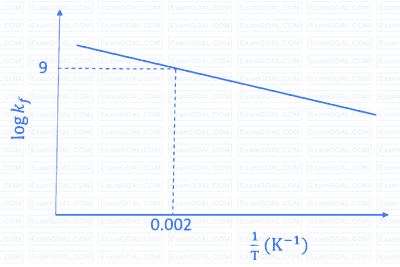
Pre-exponential factors for the forward and backward reactions are $10^{15} \mathrm{~s}^{-1}$ and $10^{11} \mathrm{~s}^{-1}$, respectively. If the value of $\log K$ for the reaction at $500 \mathrm{~K}$ is 6 , the value of $\left|\log k_b\right|$ at $250 \mathrm{~K}$ is ______.
$$ \begin{aligned} & {[K=\text { equilibrium constant of the reaction }} \\\\ & k_f=\text { rate constant of forward reaction } \\\\ & \left.k_b=\text { rate constant of backward reaction }\right] \end{aligned} $$

Pre-exponential factors for the forward and backward reactions are $10^{15} \mathrm{~s}^{-1}$ and $10^{11} \mathrm{~s}^{-1}$, respectively. If the value of $\log K$ for the reaction at $500 \mathrm{~K}$ is 6 , the value of $\left|\log k_b\right|$ at $250 \mathrm{~K}$ is ______.
$$ \begin{aligned} & {[K=\text { equilibrium constant of the reaction }} \\\\ & k_f=\text { rate constant of forward reaction } \\\\ & \left.k_b=\text { rate constant of backward reaction }\right] \end{aligned} $$
Your input ____
2
JEE Advanced 2020 Paper 1 Offline
Numerical
+4
-0

Consider the reaction,
A $$\rightleftharpoons $$ B
at 1000 K. At time t', the temperature of the system was increased to 2000 K and the system was allowed to reach equilibrium. Throughout this experiment the partial pressure of A was maintained at 1 bar. Given, below is the plot of the partial pressure of B with time. What is the ratio of the standard Gibbs energy of the reaction at 1000 K to that at 2000 K?

A $$\rightleftharpoons $$ B
at 1000 K. At time t', the temperature of the system was increased to 2000 K and the system was allowed to reach equilibrium. Throughout this experiment the partial pressure of A was maintained at 1 bar. Given, below is the plot of the partial pressure of B with time. What is the ratio of the standard Gibbs energy of the reaction at 1000 K to that at 2000 K?

Your input ____
3
JEE Advanced 2019 Paper 1 Offline
Numerical
+3
-0

For the following reaction, the equilibrium constant Kc at 298 K is 1.6 $$ \times $$ 1017.
Fe2+(aq) + S2-(aq) ⇌ FeS(s)
When equal volumes of
0.06 M Fe2+(aq) and 0.2 M S2$$ - $$(aq)
solutions are mixed, the equilibrium concentration of Fe2+(aq) is found by Y $$ \times $$ 10$$ - $$17 M. The value of Y is .................
Fe2+(aq) + S2-(aq) ⇌ FeS(s)
When equal volumes of
0.06 M Fe2+(aq) and 0.2 M S2$$ - $$(aq)
solutions are mixed, the equilibrium concentration of Fe2+(aq) is found by Y $$ \times $$ 10$$ - $$17 M. The value of Y is .................
Your input ____
4
JEE Advanced 2018 Paper 1 Offline
Numerical
+3
-0

A closed tank has two compartments $$A$$ and $$B,$$ both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is a perfect heat insulator (Figure $$1.$$). If the old partition is replaced by a new partition which can slide and conduct heat but does NOT allow the gas to leak across (Figure $$2$$), the volume (in $${m^3}$$) of the compartment A after the system attains equilibrium is ______________.


Your input ____
Questions Asked from Chemical Equilibrium (Numerical)
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus