1
JEE Advanced 2022 Paper 2 Online
Numerical
+3
-1

Concentration of $\mathrm{H}_{2} \mathrm{SO}_{4}$ and $\mathrm{Na}_{2} \mathrm{SO}_{4}$ in a solution is $1 \mathrm{M}$ and $1.8 \times 10^{-2} \mathrm{M}$, respectively. Molar solubility of $\mathrm{PbSO}_{4}$ in the same solution is $\mathrm{X} \times 10^{-\mathrm{Y}} \mathrm{M}$ (expressed in scientific notation). The value of $Y$ is ________.
[Given: Solubility product of $\mathrm{PbSO}_{4}\left(K_{s p}\right)=1.6 \times 10^{-8}$. For $\mathrm{H}_{2} \mathrm{SO}_{4}, K_{a l}$ is very large and $\left.K_{a 2}=1.2 \times 10^{-2}\right]$
Your input ____
2
JEE Advanced 2022 Paper 2 Online
Numerical
+3
-1

An aqueous solution is prepared by dissolving $0.1 \mathrm{~mol}$ of an ionic salt in $1.8 \mathrm{~kg}$ of water at $35^{\circ} \mathrm{C}$. The salt remains $90 \%$ dissociated in the solution. The vapour pressure of the solution is $59.724 \mathrm{~mm}$ of Hg. Vapor pressure of water at $35{ }^{\circ} \mathrm{C}$ is $60.000 \mathrm{~mm}$ of $\mathrm{Hg}$. The number of ions present per formula unit of the ionic salt is _________.
Your input ____
3
JEE Advanced 2022 Paper 2 Online
Numerical
+3
-1

Consider the strong electrolytes $Z_{m} X_{n}, U_{m} Y_{p}$ and $V_{m} X_{n}$. Limiting molar conductivity ( $\Lambda^{0}$ ) of $\mathrm{U}_{\mathrm{m}} \mathrm{Y}_{\mathrm{p}}$ and $\mathrm{V}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}}$ are 250 and $440 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$, respectively. The value of $(\mathrm{m}+\mathrm{n}+\mathrm{p})$ is
Given:
$\lambda^{0}$ is the limiting molar conductivity of ions
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.
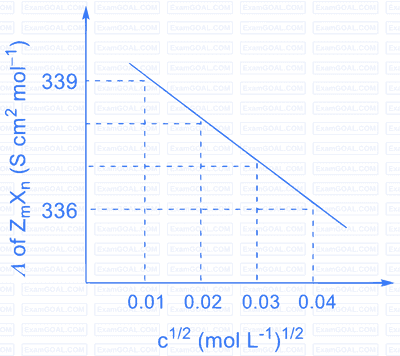
Given:
| Ion | $\mathrm{Z}^{\mathrm{n}+}$ | $\mathrm{U}^{\mathrm{p}+}$ | $\mathrm{V}^{\mathrm{n}+}$ | $\mathrm{X}^{\mathrm{m}-}$ | $\mathrm{Y}^{\mathrm{m}-}$ |
|---|---|---|---|---|---|
| $\lambda^{0}\left(\mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}\right)$ | $50.0$ | $25.0$ | $100.0$ | $80.0$ | $100.0$ |
$\lambda^{0}$ is the limiting molar conductivity of ions
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.

Your input ____
4
JEE Advanced 2022 Paper 2 Online
Numerical
+3
-1

The reaction of $\mathrm{Xe}$ and $\mathrm{O}_{2}{F}_{2}$ gives a $\mathrm{Xe}$ compound $\mathbf{P}$. The number of moles of $\mathrm{HF}$ produced by the complete hydrolysis of $1 \mathrm{~mol}$ of $\mathbf{P}$ is __________.
Your input ____
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978