1
JEE Advanced 2022 Paper 2 Online
Numerical
+3
-1

Consider the strong electrolytes $Z_{m} X_{n}, U_{m} Y_{p}$ and $V_{m} X_{n}$. Limiting molar conductivity ( $\Lambda^{0}$ ) of $\mathrm{U}_{\mathrm{m}} \mathrm{Y}_{\mathrm{p}}$ and $\mathrm{V}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}}$ are 250 and $440 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$, respectively. The value of $(\mathrm{m}+\mathrm{n}+\mathrm{p})$ is
Given:
$\lambda^{0}$ is the limiting molar conductivity of ions
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.
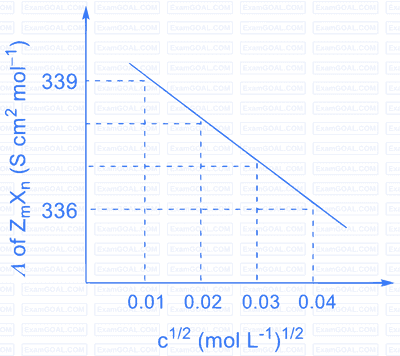
Given:
| Ion | $\mathrm{Z}^{\mathrm{n}+}$ | $\mathrm{U}^{\mathrm{p}+}$ | $\mathrm{V}^{\mathrm{n}+}$ | $\mathrm{X}^{\mathrm{m}-}$ | $\mathrm{Y}^{\mathrm{m}-}$ |
|---|---|---|---|---|---|
| $\lambda^{0}\left(\mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}\right)$ | $50.0$ | $25.0$ | $100.0$ | $80.0$ | $100.0$ |
$\lambda^{0}$ is the limiting molar conductivity of ions
The plot of molar conductivity ( $\Lambda$ ) of $\mathrm{Z}_{\mathrm{m}} \mathrm{X}_{\mathrm{n}} v s\, \mathrm{c}^{1 / 2}$ is given below.

Your input ____
2
JEE Advanced 2022 Paper 1 Online
Numerical
+3
-0

The reduction potential $\left(E^{0}\right.$, in $\left.\mathrm{V}\right)$ of $\mathrm{MnO}_{4}^{-}(\mathrm{aq}) / \mathrm{Mn}(\mathrm{s})$ is __________.
[Given: $E_{\left(\mathrm{MnO}_{4}^{-}(\mathrm{aq}) / \mathrm{MnO}_{2}(\mathrm{~s})\right)}^{0}=1.68 \mathrm{~V} ; E_{\left(\mathrm{MnO}_{2}(\mathrm{~s}) / \mathrm{Mn}^{2+}(\mathrm{aq})\right)}^{0}=1.21 \mathrm{~V} ; E_{\left(\mathrm{Mn}^{2+}(\mathrm{aq}) / \mathrm{Mn}(\mathrm{s})\right)}^{0}=-1.03 \mathrm{~V}$ ]
Your input ____
3
JEE Advanced 2021 Paper 2 Online
Numerical
+2
-0

At 298 K, the limiting molar conductivity of a weak monobasic acid is 4 $$\times$$ 102 S cm2 mol$$-$$1. At 298 K, for an aqueous solution of the acid the degree of dissociation is $$\alpha$$ and the molar conductivity is y $$\times$$ 102 S cm2 mol$$-$$1. At 298 K, upon 20 times dilution with water, the molar conductivity of the solution becomes 3y $$\times$$ 102 S cm2 mol$$-$$1.
The value of $$\alpha$$ is __________.
The value of $$\alpha$$ is __________.
Your input ____
4
JEE Advanced 2021 Paper 2 Online
Numerical
+2
-0

At 298 K, the limiting molar conductivity of a weak monobasic acid is 4 $$\times$$ 102 S cm2 mol$$-$$1. At 298 K, for an aqueous solution of the acid the degree of dissociation is $$\alpha$$ and the molar conductivity is y $$\times$$ 102 S cm2 mol$$-$$1. At 298 K, upon 20 times dilution with water, the molar conductivity of the solution becomes 3y $$\times$$ 102 S cm2 mol$$-$$1.
The value of y is __________.
The value of y is __________.
Your input ____
Questions Asked from Electrochemistry (Numerical)
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced 2025 Paper 2 Online (1)
JEE Advanced 2025 Paper 1 Online (1)
JEE Advanced 2022 Paper 2 Online (1)
JEE Advanced 2022 Paper 1 Online (1)
JEE Advanced 2021 Paper 2 Online (2)
JEE Advanced 2020 Paper 1 Offline (1)
JEE Advanced 2018 Paper 2 Offline (1)
JEE Advanced 2018 Paper 1 Offline (1)
JEE Advanced 2017 Paper 1 Offline (1)
JEE Advanced 2015 Paper 2 Offline (1)
JEE Advanced 2015 Paper 1 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus