1
JEE Advanced 2020 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-2

Consider the following four compounds, I, II, III, and IV.


Choose the correct statement(s)


Choose the correct statement(s)
2
JEE Advanced 2020 Paper 1 Offline
MCQ (More than One Correct Answer)
+4
-2

With respect to the compounds I-V, choose the correct statement(s).


3
JEE Advanced 2017 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

For the following compounds, the correct statement(s) with respect to nucleophilic substitution reaction is (are)


4
JEE Advanced 2017 Paper 1 Offline
MCQ (More than One Correct Answer)
+4
-1

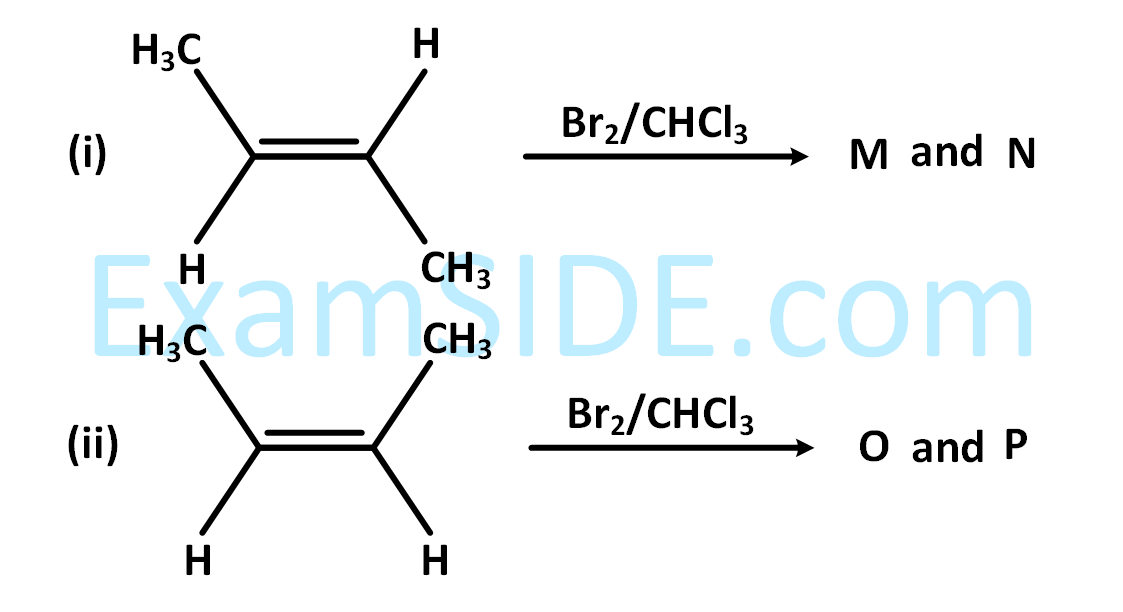
The correct statements(s) for the following addition reactions is (are)

Questions Asked from Basics of Organic Chemistry (MCQ (Multiple Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced 2020 Paper 2 Offline (1)
JEE Advanced 2020 Paper 1 Offline (1)
JEE Advanced 2017 Paper 2 Offline (1)
JEE Advanced 2017 Paper 1 Offline (2)
JEE Advanced 2014 Paper 1 Offline (1)
JEE Advanced 2013 Paper 1 Offline (1)
IIT-JEE 2012 Paper 2 Offline (1)
IIT-JEE 2012 Paper 1 Offline (1)
IIT-JEE 2011 Paper 1 Offline (1)
IIT-JEE 2010 Paper 1 Offline (1)
IIT-JEE 2009 Paper 1 Offline (1)
IIT-JEE 2008 Paper 1 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus