1
JEE Advanced 2023 Paper 1 Online
Numerical
+4
-0

A Hydrogen-like atom has atomic number $Z$. Photons emitted in the electronic transitions from level $n=4$ to level $n=3$ in these atoms are used to perform photoelectric effect experiment on a target metal. The maximum kinetic energy of the photoelectrons generated is $1.95 \mathrm{eV}$. If the photoelectric threshold wavelength for the target metal is $310 \mathrm{~nm}$, the value of $Z$ is _________.
[Given: $h c=1240 \mathrm{eV}-\mathrm{nm}$ and $R h c=13.6 \mathrm{eV}$, where $R$ is the Rydberg constant, $h$ is the Planck's constant and $c$ is the speed of light in vacuum]
[Given: $h c=1240 \mathrm{eV}-\mathrm{nm}$ and $R h c=13.6 \mathrm{eV}$, where $R$ is the Rydberg constant, $h$ is the Planck's constant and $c$ is the speed of light in vacuum]
Your input ____
2
JEE Advanced 2021 Paper 2 Online
Numerical
+4
-0

In a photoemission experiment, the maximum kinetic energies of photoelectrons from metals P, Q and R are EP, EQ and ER, respectively, and they are related by EP = 2EQ = 2ER. In this experiment, the same source of monochromatic light is used for metals P and Q while a different source of monochromatic light is used for the metal R. The work functions for metals P, Q and R are 4.0 eV, 4.5 eV and 5.5 eV, respectively. The energy of the incident photon used for metal R, in eV, is ___________.
Your input ____
3
JEE Advanced 2019 Paper 2 Offline
Numerical
+3
-0

A perfectly reflecting mirror of mass M mounted on a spring constitutes a spring-mass system of angular frequency $$\Omega $$ such that $${{4\pi M\Omega } \over h} = {10^{24}}{m^{ - 2}}$$ with h as Planck's constant. N photons of wavelength $$\lambda $$ = 8$$\pi $$ $$ \times $$ 10$$ - $$6 m strike the mirror simultaneously at normal incidence such that the mirror gets displaced by 1 $$\mu $$m. If the value of N is x $$ \times $$ 1012, then the value of x is ................ [Consider the spring as massless]
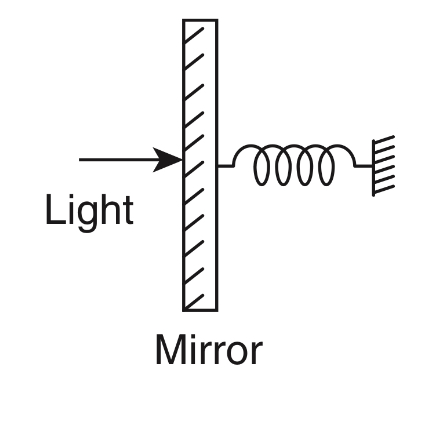

Your input ____
4
JEE Advanced 2018 Paper 2 Offline
Numerical
+3
-0

In a photoelectric experiment a parallel beam of monochromatic light with power of $$200$$ $$W$$ is incident on a perfectly absorbing cathode of work function $$6.25$$ $$ev.$$ The frequency of light is just above the threshold frequency so that the photoelectrons are emitted with negligible kinetic energy. Assume that the photoelectron emission efficiency is $$100\% $$. A potential difference of $$500$$ $$V$$ is applied between the cathode and the anode. All the emitted electrons are incident normally on the anode and are absorbed. The anode experiences a force $$F = n \times {10^{ - 4}}$$ $$N$$ due to the impact of the electrons. The value of $$n$$ is ______________. Mass of the electron $${M_e} = 9 \times {10^{ - 31}}\,kg$$ and $$1.0eV = 1.6 \times {10^{ - 19}}\,J.$$
Your input ____
Questions Asked from Dual Nature of Radiation (Numerical)
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced 2025 Paper 2 Online (1)
JEE Advanced 2025 Paper 1 Online (1)
JEE Advanced 2023 Paper 1 Online (1)
JEE Advanced 2021 Paper 2 Online (1)
JEE Advanced 2019 Paper 2 Offline (1)
JEE Advanced 2018 Paper 2 Offline (1)
JEE Advanced 2015 Paper 2 Offline (1)
JEE Advanced 2013 Paper 1 Offline (1)
IIT-JEE 2012 Paper 1 Offline (1)
IIT-JEE 2011 Paper 1 Offline (1)
IIT-JEE 2011 Paper 2 Offline (1)
IIT-JEE 2010 Paper 1 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus