1
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1

The wave function, $${\psi _{n,1,{m_1}}}$$ is a mathematical function whose value depends upon spherical polar coordinates $$\left( {r,\theta ,\phi } \right)$$ of the electron and characterized by the quantum numbers $$n,1$$ and $${m_1}$$. Here $$r$$ is distance from nucleus, $$\theta $$ is colatitude and $$\phi $$ is azimuth. In the mathematical functions given in the table, $$Z$$ is atomic number and $${a_0}$$ is Bohr radius.
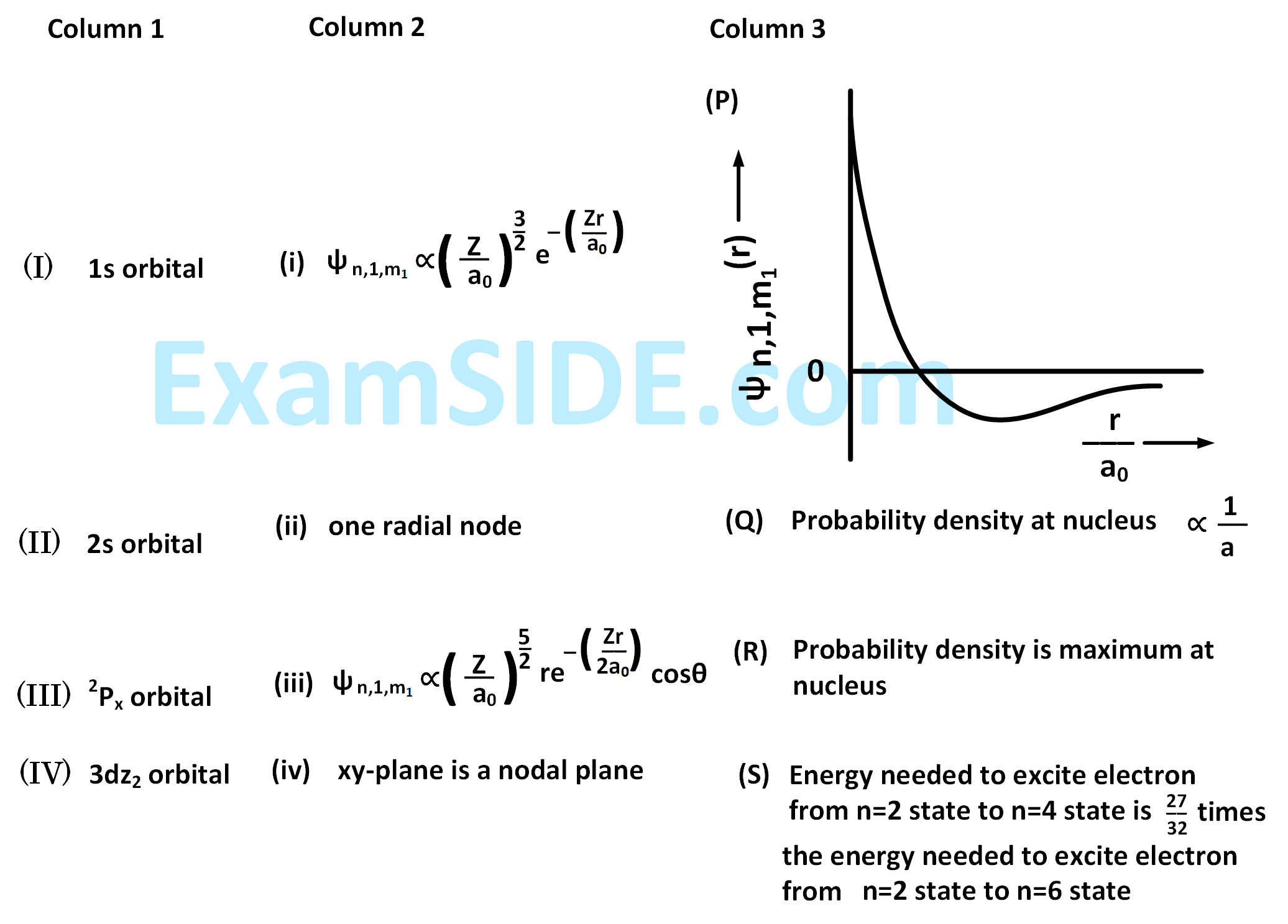

For hydrogen atom, the only CORRECT combination is :
2
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1

The wave function, $${\psi _{n,1,{m_1}}}$$ is a mathematical function whose value depends upon spherical polar coordinates $$\left( {r,\theta ,\phi } \right)$$ of the electron and characterized by the quantum numbers $$n,1$$ and $${m_1}$$. Here $$r$$ is distance from nucleus, $$\theta $$ is colatitude and $$\phi $$ is azimuth. In the mathematical functions given in the table, $$Z$$ is atomic number and $${a_0}$$ is Bohr radius.


For the given orbital in Column 1, the only CORRECT combination for any hydrogen-like species is :
3
JEE Advanced 2016 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1

P is the probability of finding the 1s electron of hydrogen atom in a spherical shell of infinitesimal thickness, dr, at a distance r from the nucleus. The volume of this shell is $$4\pi r^2dr$$. The quantitative ketch of the dependence of P on r is
4
IIT-JEE 2012 Paper 1 Offline
MCQ (Single Correct Answer)
+4
-1
The kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is [$$\alpha_0$$ is Bohr radius]
Questions Asked from Structure of Atom (MCQ (Single Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced 2024 Paper 2 Online (1)
JEE Advanced 2019 Paper 2 Offline (2)
JEE Advanced 2017 Paper 1 Offline (3)
JEE Advanced 2016 Paper 1 Offline (1)
IIT-JEE 2012 Paper 1 Offline (1)
IIT-JEE 2010 Paper 2 Offline (3)
IIT-JEE 2008 Paper 2 Offline (1)
IIT-JEE 2008 Paper 1 Offline (1)
IIT-JEE 2006 (1)
IIT-JEE 2005 Screening (1)
IIT-JEE 2004 Screening (1)
IIT-JEE 2003 Screening (1)
IIT-JEE 2002 Screening (2)
IIT-JEE 2001 Screening (2)
IIT-JEE 2000 Screening (2)
IIT-JEE 1999 (1)
IIT-JEE 1998 (2)
IIT-JEE 1997 (1)
IIT-JEE 1996 (1)
IIT-JEE 1995 Screening (1)
IIT-JEE 1992 (2)
IIT-JEE 1989 (2)
IIT-JEE 1988 (3)
IIT-JEE 1986 (4)
IIT-JEE 1985 (3)
IIT-JEE 1984 (3)
IIT-JEE 1983 (3)
IIT-JEE 1982 (1)
IIT-JEE 1981 (1)
IIT-JEE 1979 (1)
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus



