1
JEE Advanced 2023 Paper 1 Online
MCQ (Single Correct Answer)
+3
-1

In the given reaction scheme, $\mathbf{P}$ is a phenyl alkyl ether, $\mathbf{Q}$ is an aromatic compound; $\mathbf{R}$ and $\mathbf{S}$ are the major products.

The correct statement about $\mathbf{S}$ is :

The correct statement about $\mathbf{S}$ is :
2
JEE Advanced 2023 Paper 1 Online
Numerical
+4
-0

The stoichiometric reaction of $516 \mathrm{~g}$ of dimethyldichlorosilane with water results in a tetrameric cyclic product $\mathbf{X}$ in $75 \%$ yield. The weight (in g) of $\mathbf{X}$ obtained is _______.
[Use, molar mass $\left(\mathrm{g} ~\mathrm{mol}^{-1}\right): \mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{Si}=28, \mathrm{Cl}=35.5$ ]
[Use, molar mass $\left(\mathrm{g} ~\mathrm{mol}^{-1}\right): \mathrm{H}=1, \mathrm{C}=12, \mathrm{O}=16, \mathrm{Si}=28, \mathrm{Cl}=35.5$ ]
Your input ____
3
JEE Advanced 2023 Paper 1 Online
Numerical
+4
-0

A gas has a compressibility factor of 0.5 and a molar volume of $0.4 ~\mathrm{dm}^3 \mathrm{~mol}^{-1}$ at a temperature of $800 \mathrm{~K}$ and pressure $\mathbf{x}$ atm. If it shows ideal gas behaviour at the same temperature and pressure, the molar volume will be $\mathbf{y} ~\mathrm{dm}^3 \mathrm{~mol}^{-1}$. The value of $\mathbf{x} / \mathbf{y}$ is __________.
[Use: Gas constant, $\mathrm{R}=8 \times 10^{-2} \mathrm{~L}$ atm $\mathrm{K}^{-1} \mathrm{~mol}^{-1}$ ]
[Use: Gas constant, $\mathrm{R}=8 \times 10^{-2} \mathrm{~L}$ atm $\mathrm{K}^{-1} \mathrm{~mol}^{-1}$ ]
Your input ____
4
JEE Advanced 2023 Paper 1 Online
Numerical
+4
-0

The plot of $\log k_f$ versus $1 / T$ for a reversible reaction $\mathrm{A}(\mathrm{g}) \rightleftharpoons \mathrm{P}(\mathrm{g})$ is shown.
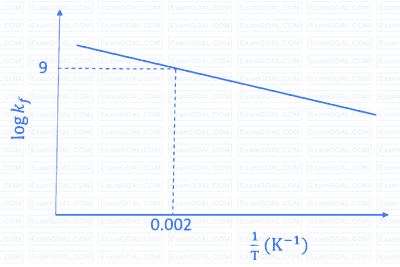
Pre-exponential factors for the forward and backward reactions are $10^{15} \mathrm{~s}^{-1}$ and $10^{11} \mathrm{~s}^{-1}$, respectively. If the value of $\log K$ for the reaction at $500 \mathrm{~K}$ is 6 , the value of $\left|\log k_b\right|$ at $250 \mathrm{~K}$ is ______.
$$ \begin{aligned} & {[K=\text { equilibrium constant of the reaction }} \\\\ & k_f=\text { rate constant of forward reaction } \\\\ & \left.k_b=\text { rate constant of backward reaction }\right] \end{aligned} $$

Pre-exponential factors for the forward and backward reactions are $10^{15} \mathrm{~s}^{-1}$ and $10^{11} \mathrm{~s}^{-1}$, respectively. If the value of $\log K$ for the reaction at $500 \mathrm{~K}$ is 6 , the value of $\left|\log k_b\right|$ at $250 \mathrm{~K}$ is ______.
$$ \begin{aligned} & {[K=\text { equilibrium constant of the reaction }} \\\\ & k_f=\text { rate constant of forward reaction } \\\\ & \left.k_b=\text { rate constant of backward reaction }\right] \end{aligned} $$
Your input ____
Paper analysis
Total Questions
Chemistry
17
Mathematics
17
Physics
17
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004
IIT-JEE 2004 Screening
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001
IIT-JEE 2001 Screening
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998
IIT-JEE 1998 Screening
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978