1
JEE Advanced 2021 Paper 1 Online
Numerical
+2
-0

For the reaction, X(s) $$\rightleftharpoons$$ Y(s) + Z(g), the plot of $$\ln {{pz} \over {{p^\theta }}}$$ versus $${{{{10}^4}} \over T}$$ is given below (in solid line), where pz is the pressure (in bar) of the gas Z at temperature T and $${{p^\theta }}$$ = 1 bar.
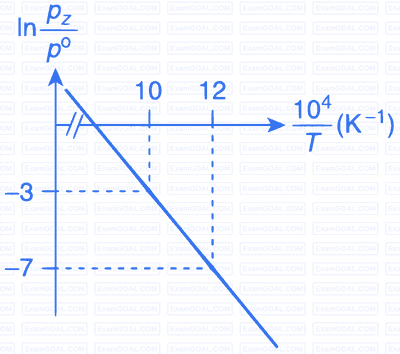
(Given, $${{d(\ln K)} \over {d\left( {{1 \over T}} \right)}} = - {{\Delta {H^\theta }} \over R}$$, where the equilibrium
constant, $$K = {{pz} \over {{p^\theta }}}$$ and the gas constant, R = 8.314 J K$$-$$1 mol$$-$$1)

(Given, $${{d(\ln K)} \over {d\left( {{1 \over T}} \right)}} = - {{\Delta {H^\theta }} \over R}$$, where the equilibrium
constant, $$K = {{pz} \over {{p^\theta }}}$$ and the gas constant, R = 8.314 J K$$-$$1 mol$$-$$1)
The value of $$\Delta$$S$$\theta$$ (in J K$$-$$1 mol$$-$$1) for the given reaction, at 1000 K is _________.
Your input ____
2
JEE Advanced 2021 Paper 1 Online
Numerical
+2
-0

The boiling point of water in a 0.1 molal silver nitrate solution (solution A) is x$$^\circ$$C. To this solution A, an equal volume of 0.1 molal aqueous barium chloride solution is added to make a new solution B. The difference in the boiling points of water in the two solutions A and B is y $$\times$$ 10$$-$$2$$^\circ$$C.
(Assume : Densities of the solutions A and B are the same as that of water and the soluble salts dissociate completely.
Use : Molal elevation constant (Ebullioscopic Constant), Kb = 0.5 K kg mol$$-$$1; Boiling point of pure water as 100$$^\circ$$C.)
(Assume : Densities of the solutions A and B are the same as that of water and the soluble salts dissociate completely.
Use : Molal elevation constant (Ebullioscopic Constant), Kb = 0.5 K kg mol$$-$$1; Boiling point of pure water as 100$$^\circ$$C.)
The value of x is ________.
Your input ____
3
JEE Advanced 2021 Paper 1 Online
Numerical
+2
-0

The boiling point of water in a 0.1 molal silver nitrate solution (solution A) is x$$^\circ$$C. To this solution A, an equal volume of 0.1 molal aqueous barium chloride solution is added to make a new solution B. The difference in the boiling points of water in the two solutions A and B is y $$\times$$ 10$$-$$2$$^\circ$$C.
(Assume : Densities of the solutions A and B are the same as that of water and the soluble salts dissociate completely.
Use : Molal elevation constant (Ebullioscopic Constant), Kb = 0.5 K kg mol$$-$$1; Boiling point of pure water as 100$$^\circ$$C.)
(Assume : Densities of the solutions A and B are the same as that of water and the soluble salts dissociate completely.
Use : Molal elevation constant (Ebullioscopic Constant), Kb = 0.5 K kg mol$$-$$1; Boiling point of pure water as 100$$^\circ$$C.)
The value of | y | is ________.
Your input ____
4
JEE Advanced 2021 Paper 1 Online
MCQ (More than One Correct Answer)
+4
-2

Given :

The compound(s), which on reaction with HNO3 will give the product having degree of rotation, [$$\alpha$$]D = $$-$$52.7$$^\circ$$ is (are)

The compound(s), which on reaction with HNO3 will give the product having degree of rotation, [$$\alpha$$]D = $$-$$52.7$$^\circ$$ is (are)
Paper analysis
Total Questions
Chemistry
19
Mathematics
19
Physics
19
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004
IIT-JEE 2004 Screening
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001
IIT-JEE 2001 Screening
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998
IIT-JEE 1998 Screening
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978



