1
JEE Advanced 2025 Paper 2 Online
Numerical
+4
-0

A hydrogen atom, initially at rest in its ground state, absorbs a photon of frequency $v_1$ and ejects the electron with a kinetic energy of 10 eV . The electron then combines with a positron at rest to form a positronium atom in its ground state and simultaneously emits a photon of frequency $v_2$. The center of mass of the resulting positronium atom moves with a kinetic energy of 5 eV . It is given that positron has the same mass as that of electron and the positronium atom can be considered as a Bohr atom, in which the electron and the positron orbit around their center of mass. Considering no other energy loss during the whole process, the difference between the two photon energies (in eV) is ____________.
Your input ____
2
JEE Advanced 2025 Paper 2 Online
Numerical
+4
-0

An ideal monatomic gas of $n$ moles is taken through a cycle $W X Y Z W$ consisting of consecutive adiabatic and isobaric quasi-static processes, as shown in the schematic $V-T$ diagram. The volume of the gas at $W, X$ and $Y$ points are, $64 \mathrm{~cm}^3, 125 \mathrm{~cm}^3$ and $250 \mathrm{~cm}^3$, respectively. If the absolute temperature of the gas $T_W$ at the point $W$ is such that $n R T_W=1 \mathrm{~J}$ ( $R$ is the universal gas constant), then the amount of heat absorbed (in J ) by the gas along the path $X Y$ is ___________.
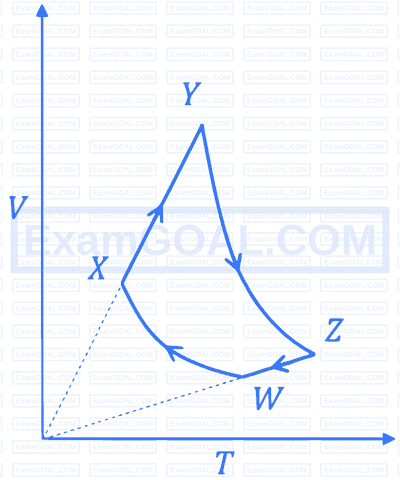

Your input ____
3
JEE Advanced 2025 Paper 2 Online
Numerical
+4
-0

A geostationary satellite above the equator is orbiting around the earth at a fixed distance $r_1$ from the center of the earth. A second satellite is orbiting in the equatorial plane in the opposite direction to the earth's rotation, at a distance $r_2$ from the center of the earth, such that $r_1=1.21 r_2$. The time period of the second satellite as measured from the geostationary satellite is $\frac{24}{p}$ hours. The value of $p$ is _________.
Your input ____
4
JEE Advanced 2025 Paper 2 Online
Numerical
+4
-0

The left and right compartments of a thermally isolated container of length $L$ are separated by a thermally conducting, movable piston of area $A$. The left and right compartments are filled with $\frac{3}{2}$ and 1 moles of an ideal gas, respectively. In the left compartment the piston is attached by a spring with spring constant $k$ and natural length $\frac{2 L}{5}$. In thermodynamic equilibrium, the piston is a distance $\frac{L}{2}$ from the left and right edges of the container as shown in the figure. Under the above conditions, if the pressure in the right compartment is $P=\frac{k L}{A} \alpha$, then the value of $\alpha$ is __________.
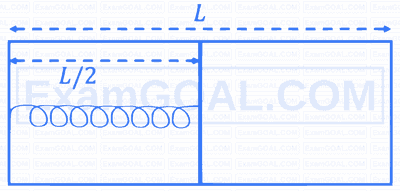

Your input ____
Paper analysis
Total Questions
Chemistry
16
Mathematics
16
Physics
16
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978