1
JEE Advanced 2019 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

A thin and uniform rod of mass M and length L is held vertical on a floor with large friction. The rod is released from rest so that it falls by rotating about its contact-point with the floor without slipping. Which of the following statement(s) is/are correct, when the rod makes an angle 60$$^\circ $$ with vertical? [g is the acceleration due to gravity]
2
JEE Advanced 2019 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

In a Young's double slit experiment, the slit separation d is 0.3 mm and the screen distance D is 1 m. A parallel beam of light of wavelength 600 nm is incident on the slits at angle $$\alpha $$ as shown in figure.
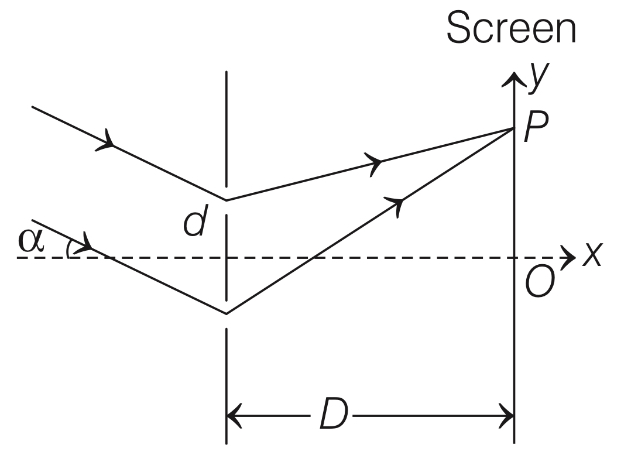
On the screen, the point O is equidistant from the slits and distance PO is 11.0 mm. Which of the following statement(s) is/are correct?

On the screen, the point O is equidistant from the slits and distance PO is 11.0 mm. Which of the following statement(s) is/are correct?
3
JEE Advanced 2019 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

A free hydrogen atom after absorbing a photon of wavelength $$\lambda $$a gets excited from the state n = 1 to the state n = 4. Immediately after that the electron jumps to n = m state by emitting a photon of wavelength $$\lambda $$e. Let the change in momentum of atom due to the absorption and the emission be $$\Delta {p_a}$$ and $$\Delta {p_e}$$, respectively. If $${{{\lambda _a}} \over {{\lambda _e}}} = {1 \over 5}$$, which of the option(s) is/are correct? [Use hc = 1242 eVnm; 1 nm = 10-9 m, h and c are Planck's constant and speed of light in vacuum, respectively]
4
JEE Advanced 2019 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

A mixture of ideal gas containing 5 moles of monatomic gas and 1 mole of rigid diatomic gas is initially at pressure P0, volume V0, and temperature T0. If the gas mixture is adiabatically compressed to a volume $${{{V_0}} \over 4}$$, then the correct statement(s) is/are
(Given, 21.2 = 2.3; 23.2 = 9.2; R is a gas constant)
(Given, 21.2 = 2.3; 23.2 = 9.2; R is a gas constant)
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978