1
JEE Advanced 2019 Paper 2 Offline
Numerical
+3
-0

The mole fraction of urea in an aqueous urea solution containing 900 g of water is 0.05. If the density of the solution is 1.2 g cm$$-$$3, then molarity of urea solution is ................
(Given data : Molar masses of urea and water are 60 g mol$$-$$1 and 18 g mol$$-$$1, respectively)
(Given data : Molar masses of urea and water are 60 g mol$$-$$1 and 18 g mol$$-$$1, respectively)
Your input ____
2
JEE Advanced 2019 Paper 2 Offline
Numerical
+3
-0

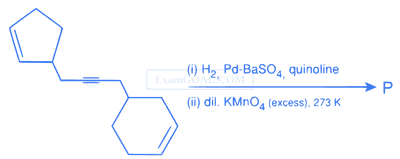
Total number of hydroxyl groups present in a molecule of the major product P is ..............

Your input ____
3
JEE Advanced 2019 Paper 2 Offline
Numerical
+3
-0

Total number of $$cis\,N - Mn - Cl$$ bond angles (that is $$Mn - N$$ and $$Mn - Cl$$ bonds in cis positions) present in a molecule of $$cis[Mn{(en)_2}C{l_2}]$$ complex is ..................
(en = NH2CH2CH2NH2)
(en = NH2CH2CH2NH2)
Your input ____
4
JEE Advanced 2019 Paper 2 Offline
Numerical
+3
-0

The amount of water produced (in g) in the oxidation of 1 mole of rhombic sulphur by conc. HNO3 to a compound with the highest oxidation state of sulphur is ..............
(Given data : Molar mass of water = 18 g mol$$-$$1)
(Given data : Molar mass of water = 18 g mol$$-$$1)
Your input ____
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978