1
GATE ME 2017 Set 2
MCQ (Single Correct Answer)
+2
-0.6
The volume and temperature of air (assumed to be an ideal gas) in a closed vessel is $$2.87{m^3}$$ and $$300$$ $$K,$$ respectively. The gauge pressure indicated by a manometer fitted to the wall of the vessel is $$0.5$$ bar. If the gas constant of air is
$$R =287J/kgK$$ and the atmospheric pressure is $$1$$ bar, the mass of air (in $$kg$$) in the vessel is
$$R =287J/kgK$$ and the atmospheric pressure is $$1$$ bar, the mass of air (in $$kg$$) in the vessel is
2
GATE ME 2015 Set 1
Numerical
+2
-0
Temperature of nitrogen in a vessel of volume $$2{m^3}$$ is $$288$$ $$K.$$ $$A$$ $$U$$-tube manometer connected to the vessel shows a reading of $$70$$ $$cm$$ of mercury (level higher in the end open to atmosphere). The universal gas constant is $$8314$$ $$J/kmol$$-$$K,$$ atmospheric pressure is $$1.01325$$ bar, acceleration due to gravity is $$9.81$$ $$m/{s^2}$$ and density of mercury is $$13600$$ $$kg/{m^3}.$$ The mass of nitrogen (in $$kg$$) in the vessel is _________
Your input ____
3
GATE ME 2014 Set 3
MCQ (Single Correct Answer)
+2
-0.6
A certain amount of an ideal gas is initially at a pressure $${p_1}$$ and temperature $${T_1}$$. First, it undergoes a constant pressure process $$1$$-$$2$$ such that $$T{}_2 = 3{T_1}/4.$$ Then, it undergoes a constant volume process $$2$$-$$3$$ such that $${T_3} = {T_1}/2.$$ The ratio of the final volume to the initial volume of the ideal gas is
4
GATE ME 2006
MCQ (Single Correct Answer)
+2
-0.6
Match items from groups, $${\rm I},$$ $${\rm II},$$ $${\rm III},$$ $${\rm IV}$$ and $$V$$

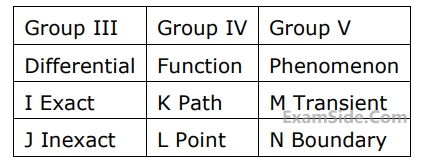


Questions Asked from Basic Concepts and Zeroth Law (Marks 2)
Number in Brackets after Paper Indicates No. of Questions
GATE ME Subjects
Engineering Mechanics
Machine Design
Strength of Materials
Heat Transfer
Production Engineering
Industrial Engineering
Turbo Machinery
Theory of Machines
Engineering Mathematics
Fluid Mechanics
Thermodynamics
General Aptitude