Marks 1
Marks 2
Consider a mixture of two ideal gases, X and Y, with molar masses M̅X = 10 kg/kmol and M̅Y = 20 kg/kmol, respectively, in a container. The total pressure in the container is 100 kPa, the total volume of the container is 10 m3 and the temperature of the contents of the container is 300 K. If the mass of gas-X in the container is 2 kg, then the mass of gas-Y in the container is ____ kg. (Rounded off to one decimal place)
Assume that the universal gas constant is 8314 J kmol-1K-1.
$$R =287J/kgK$$ and the atmospheric pressure is $$1$$ bar, the mass of air (in $$kg$$) in the vessel is

Assume that the volume of the football remains constant at $$2500c{m^3}$$
The amount of heat lost by the air in the football and the gauge pressure of air in the football at the stadium respectively equal
Assume that the volume of the football remains constant at $$2500c{m^3}$$
Gauge pressure of air to which the ball must have been originally inflated so that it would equal $$1$$ bar gauge at the stadium is
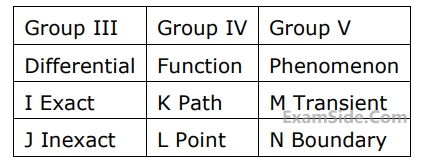
List - $${\rm I}$$
$$A.$$ Cetane number
$$B.$$ Approach and range
$$C.$$ $${\left( {{{\delta T} \over {\delta P}}} \right)_h} \ne 0$$
$$D.$$ $$dh = {c_p}\,\,dT,$$ even when pressure varies
List - $${\rm II}$$
$$1.$$ Ideal gas
$$2.$$ Vander Waals gas
$$3.$$ $$S.\,{\rm I}.$$ engine
$$4.$$ $$C.\,{\rm I}.$$ engine
$$5.$$ Cooling towers
$$6.$$ Heat exchangers