Properties of Pure Substances · Thermodynamics · GATE ME
Marks 1
$$\left( {{V_f} = 0.00106\,{m^3}/kg,\,\,{v_g} = 0.8908\,\,{m^3}/kg} \right).$$ The state of water is
Marks 2
Heat loss to the surrounding is $$50$$ $$kJ/kg$$ of steam flowing through the turbine. Neglecting changes in kinetic energy and potential energy, the work output of the turbine (in $$kJ/kg$$ of steam) is _____________

Specific volume of liquid $$\left( {{v_f}} \right)$$ and vapour $$\left( {{v_g}} \right)$$ phases, as well as values of saturation temperatures, are given in the table below.

The work done by the system during the process is

Specific volume of liquid $$\left( {{v_f}} \right)$$ and vapour $$\left( {{v_g}} \right)$$ phases, as well as values of saturation temperatures, are given in the table below.

At the end of the process, which one of the following situations will be true?

Specific volume of liquid $$\left( {{v_f}} \right)$$ and vapour $$\left( {{v_g}} \right)$$ phases, as well as values of saturation temperatures, are given in the table below.

The net entropy generation (considering the system and the thermal reservoir together) during the process is closest to
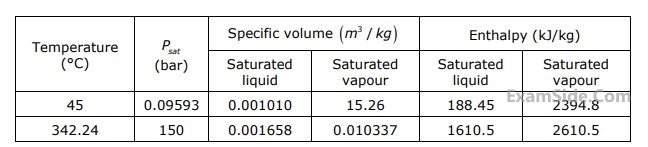
Specific enthalpy of water in $$kJ/kg$$ at $$150$$ bar and $$ {45^ \circ }C$$ is

The specific enthalpy data are in columns

When saturated liquid at $${40^ \circ }C$$ is throttled to $$ - {20^ \circ }C,$$ the quality at exit will be
$$\eqalign{ & \ln \,\,P = 15.16 - 3063/T \cr & \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,and \cr & \ln \,\,P = 18.70 - 3754/T \cr} $$
Where $$p$$ is in atmospheres and $$T$$ is in Kelvin. What is the temperature at the triple point.