
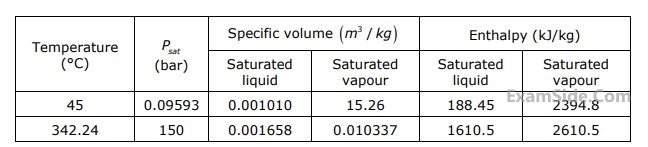
Specific volume of liquid $$\left( {{v_f}} \right)$$ and vapour $$\left( {{v_g}} \right)$$ phases, as well as values of saturation temperatures, are given in the table below.

The work done by the system during the process is

Specific volume of liquid $$\left( {{v_f}} \right)$$ and vapour $$\left( {{v_g}} \right)$$ phases, as well as values of saturation temperatures, are given in the table below.

At the end of the process, which one of the following situations will be true?

Specific volume of liquid $$\left( {{v_f}} \right)$$ and vapour $$\left( {{v_g}} \right)$$ phases, as well as values of saturation temperatures, are given in the table below.

The net entropy generation (considering the system and the thermal reservoir together) during the process is closest to
Specific enthalpy of water in $$kJ/kg$$ at $$150$$ bar and $$ {45^ \circ }C$$ is