Heat and Thermodynamics · Physics · MHT CET
MCQ (Single Correct Answer)
In case of free expansion, which one of the following statements is WRONG?
An ideal gas expands adiabatically, $(\gamma=1.5)$. To reduce the r.m.s. velocity of the molecules 4 times, the gas has to be expanded
The temperature at which oxygen molecules will have same r.m.s. speed as helium molecules at $57^{\circ} \mathrm{C}$ is (molecular masses of oxygen and helium are 32 and 4 respectively.)
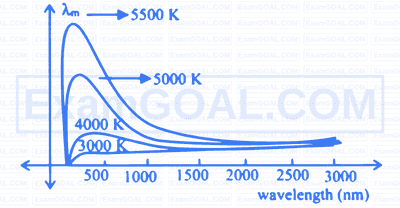
Two black spheres $\mathrm{P} \& \mathrm{Q}$ have radii in the ratio $4: 3$. The wavelength of maximum intensity of radiation are in the ratio $4: 5$ respectively. The ratio of radiated power by P to Q is
The heat energy that must be supplied to 14 gram of nitrogen at room temperature to raise its temperature by $48^{\circ} \mathrm{C}$ at constant pressure is (Molecular weight of nitrogen $=28, R=$ gas constant, $\mathrm{C}_{\mathrm{p}}=\frac{7}{2} \mathrm{R}$ for diatomic gas)
The difference in length between two rods A and B is 60 cm at all temperatures. If $\alpha_{\mathrm{A}}=18 \times 10^{-6} /{ }^{\circ} \mathrm{C}$ and $\alpha_{\mathrm{B}}=27 \times 10^{-6} /{ }^{\circ} \mathrm{C}$, then the length of $\operatorname{rod} \mathrm{A}$ and $\operatorname{rod} \mathrm{B}$ at $0^{\circ} \mathrm{C}$ is respectively
A sample of an ideal gas $\left(\gamma=\frac{5}{3}\right)$ is heated at constant pressure. If 100 J of heat is supplied to the gas, the work done by the gas is
A balloon is filled at $27^{\circ} \mathrm{C}$ and 1 atmospheric pressure by volume $500 \mathrm{~m}^3$ helium gas. At $-3^{\circ} \mathrm{C}$ and 0.5 atmospheric pressure, the volume of helium gas will be
The volume of a metal sphere increases by $0.33 \%$ when its temperature is raised by $50^{\circ} \mathrm{C}$. The coefficient of linear expansion of the metal is
Heat supplied $d Q=$ increased in internal energy dU is true for
A rectangular black body of temperature $127^{\circ} \mathrm{C}$ has surface area $4 \mathrm{~cm} \times 2 \mathrm{~cm}$ and rate of radiation is E . If its temperature is increased by $400^{\circ} \mathrm{C}$ and surface area is reduced to half of the initial value then the rate of radiation is
The temperature of an ideal gas is increased from 100 K to 400 K . If ' $x$ ' is the root mean square velocity of its molecules at 100 K , r.m.s. velocity becomes
According to the kinetic theory of gases, when two molecules of a gas collide with each other then
During the isothermal expansion, a confined ideal gas does $(-150) \mathrm{J}$ of work against its surroundings. This means that
A body cools from $80^{\circ} \mathrm{C}$ to $50^{\circ} \mathrm{C}$ in 5 min . In the next time of ' t ' in, the body continues to cool from $50^{\circ} \mathrm{C}$ to $30^{\circ} \mathrm{C}$. The total time taken by the body to cool from $80^{\circ} \mathrm{C}$ to $30^{\circ} \mathrm{C}$ is [The temperature of the surroundings is $20^{\circ} \mathrm{C}$.]
The volume of given mass of a gas is increased by $7 \%$ at constant temperature. The pressure should be increased by
A monoatomic ideal gas is compressed adiabatically to $\left(\frac{1}{27}\right)$ of its initial volume. If initial temperature of the gas is ' T ' K and final temperature is ' xT ' K , the value of ' x ' is
Select the correct statement.
A polyatomic gas at pressure P , having volume ' $V$ ' expands isothermally to a volume ' 3 V ' and then adiabatically to a volume ' 24 V '. The final pressure of gas is (for moderate temperature changes)
A stationary object at $4^{\circ} \mathrm{C}$ and weighing 3.5 kg falls from a height of 2000 m on snow mountain at $0^{\circ} \mathrm{C}$. If the temperature of the object just before hitting the snow is $0^{\circ} \mathrm{C}$ and the object comes to rest immediately then the quantity of ice that melts is (Acceleration due to gravity $=10 \mathrm{~m} / \mathrm{s}^2$, Latent heat of ice $=3.5 \times 10^5 \mathrm{~J} / \mathrm{kg}$ )
Six molecules of a gas in container have speeds $2 \mathrm{~m} / \mathrm{s}, 5 \mathrm{~m} / \mathrm{s}, 3 \mathrm{~m} / \mathrm{s}, 6 \mathrm{~m} / \mathrm{s}, 3 \mathrm{~m} / \mathrm{s}$, and $5 \mathrm{~m} / \mathrm{s}$. The r.m.s. speed is
During thermodynamic process, the increase in internal energy of a system is equal to the $\mathrm{w}_{0 r k}$ done on the system. Which process does the system undergo?
How much should the pressure be increased in order to reduce the volume of a given mass of gas by $5 \%$ at the constant temperature?
A polyatomic gas is compressed to $\left(\frac{1}{8}\right)^{\text {th }}$ of its volume adiabatically. If its initial pressure is $\mathrm{P}_0$, its new pressure will be [Given, $\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}=\frac{4}{3}$ ]
The pressure ' P ', volume ' V ' and temperature ' T ' of a gas in a jar ' $A$ ' and the gas in other jar ' $B$ ' is at pressure ' 2 P ', volume ' V ' and temperature ' $\frac{T}{4}$ '. Then the ratio of the number of molecules in jar A and jar B will be
Two moles of an ideal monoatomic gas undergo a cyclic process as shown in figure. The temperatures in different states are given as $6 \mathrm{~T}_1=3 \mathrm{~T}_2=2 \mathrm{~T}_4=\mathrm{T}_3=2400 \mathrm{~K}$. The work done by the gas during the complete cycle is ( $\mathrm{R}=$ Universal gas constant)

Two spherical black bodies have radii ' $R_1$ ' and ' $R_2$ '. Their surface temperatures are $T_1 K$ and $T_2 K$ respectively. If they radiate the same power, the ratio $\frac{R_1}{R_2}$ is
A thermometer bulb has volume $10^{-6} \mathrm{~m}^3$ and cross-section of the stem is $0.002 \mathrm{~cm}^2$. The bulb is filled with mercury at $0^{\circ} \mathrm{C}$. If the thermometer reads temperature as $100^{\circ} \mathrm{C}$, then the length of mercury column is (coefficient of cubical expansion of mercury $=18 \times 10^{-5} /{ }^{\circ} \mathrm{C}$ )
The two ends of a rod of length ' $x$ ' and uniform cross-sectional area ' A ' are kept at temperatures ' $\mathrm{T}_1$ ' and ' $\mathrm{T}_2$ ' respectively ( $\mathrm{T}_1>\mathrm{T}_2$ ). If the rate of heat transfer is ' $\mathrm{Q} / \mathrm{t}$ ', through the rod in steady state, then the coefficient of thermal conductivity ' K ' is
When the pressure of the gas contained in a closed vessel is increased by $2.3 \%$, the temperature of the gas increases by 4 K . The initial temperature of the gas is
Black bodies A and B radiate maximum energy with wavelength difference $4 \mu \mathrm{~m}$. The absolute temperature of body A is 3 times that of B. The wavelength at which body $B$ radiates maximum energy is
A monoatomic ideal gas, initially at temperature $\mathrm{T}_1$ is enclosed in a cylinder fitted with massless, frictionless piston. By releasing the piston suddenly, the gas is allowed to expand adiabatically to a temperature $\mathrm{T}_2$. If $\mathrm{L}_1$ and $\mathrm{L}_2$ are the lengths of the gas columns before and after expansion respectively, then $\left(T_2 / T_1\right)$ is given by
Two bodies A and B at temperatures ' $\mathrm{T}_1$ ' K and ' $\mathrm{T}_2$ ' K respectively have the same dimensions. Their emissivities are in the ratio $16: 1$. At $\mathrm{T}_1=\mathrm{xT}_2$, they radiate the same amount of heat per unit area per unit time. The value of $x$ is
In an isobaric process of an ideal gas, the ratio of heat supplied and work done by the system $\left(\frac{\mathrm{Q}}{\mathrm{W}}\right)$ is $\left[\frac{\mathrm{C}_{\mathrm{P}}}{\mathrm{C}_{\mathrm{V}}}=\gamma\right]$.
The temperature of a body on Kelvin scale is ' $x$ ' $K$. When it is measured by a Fahrenheit thermometer, it is found to be ' x ' ${ }^{\circ} \mathrm{F}$. The value of ' $x$ ' is (nearly)
For a gas at a particular temperature on an average, the quantity which remains same for all molecules is
If 120 J of thermal energy is incident on area $3 \mathrm{~m}^2$, the amount of heat transmitted is 12 J , coefficient of absorption is 0.6 , then the amount of heat reflected is
When an ideal gas $\left(\gamma=\frac{5}{3}\right)$ is heated under constant pressure, then what percentage of given heat energy will be utilised in doing external work?
The mean kinetic energy of the molecules of an ideal gas at $399^{\circ} \mathrm{C}$ is ' E '. The temperature at which the mean kinetic energy of its molecules will be ' $\mathrm{E} / 2$ ', is
A gas undergoes a change in which its pressure ' P ' and volume ' V ' are related as $\mathrm{PV}^{\mathrm{n}}=$ constant, where n is a constant. If the specific heat of the gas in this change is zero, then the value of $n$ is ( $\gamma=$ adiabatic ratio)
Hot water cools from $80^{\circ} \mathrm{C}$ to $60^{\circ} \mathrm{C}$ in 1 minutes. In cooling from $60^{\circ} \mathrm{C}$ to $50^{\circ} \mathrm{C}$ it will take (room temperature $=30^{\circ} \mathrm{C}$ )
A Carnot engine has efficiency $\frac{1}{6}$. It becomes $\frac{1}{3}$, when the temperature of $\operatorname{sink}$ is lowered by
57 K . The temperature of the source is
The r.m.s. speed of gas molecules at 800 K will be
If a black body at 400 K surrounded by atmosphere at 300 K has rate of cooling ' $\mathrm{R}_0$ ', the same body at 900 K , surrounded by same atmosphere, will have rate of cooling nearly
The temperature of an ideal gas is increased from 100 K to 400 K . If ' $x$ ' is the R.M.S. velocity of its molecules at 100 K , it becomes
Heat is given to an ideal gas in an isothermal process. Then
A. internal energy of the gas will decrease.
B. internal energy of the gas will increase.
C. internal energy of the gas will not change.
D. the gas will do negative work.
A rectangular block of surface area A emits energy E per second at $27^{\circ} \mathrm{C}$. If length and breadth is reduced to half of initial value and temperature is raised to $327^{\circ} \mathrm{C}$ then energy emitted per second becomes
When a diatomic gas (rigid) undergoes adiabatic change, its pressure $(\mathrm{P})$ and temperature $(\mathrm{T})$ are related as $P \propto T^c$. The value of $c$ is
For an ideal gas, the density of the gas is $\rho_0$ when temperature and pressure of the gas are $\mathrm{T}_0$ and $P_0$ respectively. when the temperature of the gas is $2 \mathrm{~T}_0$, its pressure becomes $3 \mathrm{P}_0$. The new density will be
A centigrade and Fahrenheit thermometer are dipped in boiling water. The water temperature is lowered until the Fahrenheit temperature observed is $140^{\circ} \mathrm{F}$. At that time the temperature registered by the centigrade thermometer is
An engine operating between temperatures $T_1$ and $T_2$ has efficiency $\frac{1}{5}$. When $T_2$ is lowered by 45 K , its efficiency becomes $\frac{1}{2}$. Temperatures $T_1$ and $T_2$ are respectively
Two cylinders A and B fitted with pistons contain equal amount of an ideal rigid diatomic gas at 303 K . The piston of cylinder $A$ is free to move and that of cylinder B is held fixed. The same amount heat is given to the gas in each cylinder. If the rise in temperature of the gas in cylinder B is 49 K , then the rise in temperature of the gas in $A$ is
If a gas is compressed isothermally then the r.m.s. velocity of its molecules
In a cyclic process, work done by the system is
A metal sphere cools at a rate of $1.5^{\circ} \mathrm{C} / \mathrm{min}$ when its temperature is $80^{\circ} \mathrm{C}$. When the temperature of the sphere is $40^{\circ} \mathrm{C}$, its rate of cooling is $0.3^{\circ} \mathrm{C} / \mathrm{min}$. The temperature of the surrounding $\left(\theta_0\right)$ is
The change in the internal energy of the mass of gas, when the volume changes from V to 2 V at constant pressure P is $\left(\gamma=\frac{\mathrm{Cp}}{\mathrm{Cv}}\right)$
For a perfectly black body, coefficient of emission is
A body cools from $60^{\circ} \mathrm{C}$ to $40^{\circ} \mathrm{C}$ in 6 minutes. After next 6 minutes its temperature will be (Temperature of the surroundings is $10^{\circ} \mathrm{C}$ )
A tyre of a vehicle is filled with air having pressure 270 kPa at $27^{\circ} \mathrm{C}$. The air pressure in the tyre when the temperature increases to $37^{\circ} \mathrm{C}$ is
A diatomic gas $\left(\gamma=\frac{7}{5}\right)$ is compressed adiabatically to volume $\frac{\mathrm{V}_0}{32}$, where $\mathrm{V}_0$ is its initial volume. The initial temperature of the gas is $\mathrm{T}_{\mathrm{i}}$ in kelvin and the final temperature is $\mathrm{xT}_{\mathrm{i}}$ in kelvin. The value of $x$ is
The work done by a gas as it is taken in a cyclic process (shown in graph) is

Two gases A and B are at absolute temperatures 350 K and 420 K respectively. The ratio of average kinetic energy of the molecules of gas $B$ to that of gas A is
A composite slab consists of two materials having coefficients of thermal conductivity K and $2 K$, thickness $x$ and $4 x$ respectively. The temperatures of two outer surfaces of a composite slab are $\mathrm{T}_2$ and $\mathrm{T}_1$ respectively $\left(\mathrm{T}_2>\mathrm{T}_1\right)$. The rate of heat transfer through the slab in a steady state is $\left[\frac{A\left(T_2-T_1\right) K}{x}\right] f$, where $f$ is equal to
The co-efficient of absorption and the coefficient of reflection of a thin uniform plate are 0.77 and 0.17 respectively. If 250 kcal of heat is incident on the surface of the plate, the quantity of heat transmitted is
An ideal gas at pressure ' P ' and temperature ' T ' is enclosed in a vessel of volume ' $V$ '. Some gas leaks through a hole from the vessel and the pressure of the enclosed gas falls to ' P '. Assuming that the temperature ture of the gas remains constant during the leakage , the number of moles of the gas that have leaked is
If r.m.s. velocity of hydrogen molecules is 4 times that of an oxygen molecule at $47^{\circ} \mathrm{C}$, the temperature of hydrogen molecules is (Molecular weight of Hydrogen and Oxygen are 2 and 32 respectively)
A monoatomic ideal gas is heated at constant pressure. The percentage of total heat used in increasing the internal energy and that used for doing external work is $A$ and $B$ respectively. Then the ratio, $\mathrm{A}: \mathrm{B}$ is
During an experiment, an ideal gas is found to obey an additional law $\mathrm{VP}^2=$ constant. The gas is initially at temperature ' T ' and volume ' V '. What will be the temperature of the gas when it expands to a volume 2 V ?
The first operation involved in a Carnot cycle is
Temperature remaining constant, the pressure of gas is decreased by $20 \%$. The percentage change in volume
At certain temperature, $\operatorname{rod} \mathrm{A}$ and $\operatorname{rod} \mathrm{B}$ of different materials have lengths $\mathrm{L}_{\mathrm{A}}$ and $\mathrm{L}_B$ respectively. Their co-efficients of linear expansion are $\alpha_A$ and $\alpha_B$ respectively. It is observed that the difference between their lengths remain constant at all temperatures. The ratio $L_A / L_B$ is given by
A monoatomic ideal gas is heated at constant pressure. The percentage of total heat used in changing the internal energy is
The ratio of the specific heats $\frac{C_p}{C_v}=\gamma$, in terms of degrees of freedom ( n ) is
Assuming the expression for the pressure exerted by the gas, it can be shown that pressure is
If heat energy $\Delta \mathrm{Q}$ is supplied to an ideal diatomic gas, the increase in internal energy is $\Delta U$ and the amount of work done by the gas is $\Delta \mathrm{W}$. The ratio $\Delta \mathrm{W}: \Delta \mathrm{U}: \Delta \mathrm{Q}$ is
The power radiated by a black body is P and it radiates maximum energy around the wavelength $\lambda_0$. Now the temperature of the black body is changed so that it radiates maximum energy around wavelength $\left(\frac{\lambda_0}{2}\right)$. The power radiated by it will now increase by a factor of
A bucket full of hot water is kept in a room. If it cools from $75^{\circ} \mathrm{C}$ to $70^{\circ} \mathrm{C}$ in $t_1$ minutes, from $70^{\circ} \mathrm{C}$ to $65^{\circ} \mathrm{C}$ in $\mathrm{t}_2$ minutes and $65^{\circ} \mathrm{C}$ to $60^{\circ} \mathrm{C}$ in $t_3$ minutes, then
An ideal diatomic gas is heated at constant pressure. What is the fraction of total energy applied, which increases the internal energy for the gas?
In ideal gas of $27^{\circ} \mathrm{C}$ is compressed adiabatically to $(8 / 27)$ of its original volume. If $\gamma=\frac{5}{3}$, the rise in temperature of a gas is
A cylindrical rod is having temperatures $\theta_1$ and $\theta_2$ at its ends. The rate of heat flow is $\mathrm{Q} J / \mathrm{S}$. All the linear dimensions of the rod are doubled by keeping the temperature constant. The new rate of flow of heat is
A monoatomic ideal gas, initially at temperature $T_1$ is enclosed in a cylinder fitted with frictionless piston. The gas is allowed to expand adiabatically to a temperature $T_2$ by releasing the piston suddenly. $L_1$ and $L_2$ are the lengths of the gas columns before and after the expansion respectively. The ratio $T_2 / T_1$ is
In an ideal gas at temperature $T$, the average force that a molecule applies on the walls of a closed container depends on $T$ as $\mathrm{T}^{\mathrm{x}}$. The value of $x$ is
Heat engine operating between temperature $T_1$ and $T_2$ has efficiency $\frac{1}{6}$. When $T_2$ is lowered by 62 K , its efficiency increases to $\frac{1}{3}$. Then $T_1$ and $T_2$ respectively are
The absolute temperature of a gas is determined by
When a system is taken from state ' $a$ ' to state ' $c$ ' along a path abc, it is found that $\mathrm{Q}=80 \mathrm{cal}$ and $\mathrm{W}=35 \mathrm{cal}$. Along path adc $\mathrm{Q}=65 \mathrm{cal}$ the work done W along path adc is

The ratio of work done by an ideal rigid diatomic gas to the heat supplied by the gas in an isobaric process is
The internal energy of an ideal diatomic gas corresponding to volume ' $V$ ' and pressure ' P ' is 2.5 PV. The gas expands from 1 litre to 2 litre at a constant pressure of $10^5 \mathrm{~N} / \mathrm{m}^2$. The heat supplied to a gas is
Four moles of hydrogen, two moles of helium and one mole of water vapour form an ideal gas mixture. $\left[C_{\mathrm{v}}\right.$ for hydrogen $=\frac{5}{2} R, C_v$ for helium $=\frac{3}{2} R, \quad C_{\mathrm{v}}$ for water vapour $\left.=3 \mathrm{R}\right]$ What is the molar specific heat at constant pressure of the mixture?
A sheet of steel is 40 cm long and 5 cm broad at $0^{\circ} \mathrm{C}$. The surface area of the sheet increases by $1.4 \mathrm{~cm}^2$ at $100^{\circ} \mathrm{C}$. Coefficient of linear expansion of steel is
A quantity of heat ' $Q$ ' is supplied to monoatomic ideal gas which expands at constant pressure. The fraction of heat converted into work is $\left[\gamma=\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}=\frac{5}{3}\right]$
What is the pressure of hydrogen in a cylinder of volume 10 litre if its total energy of translation is $7.5 \times 10^3 \mathrm{~J}$ ?
' $N$ ' molecules of gas $A$, each having mass ' $m$ ' and ' 2 N ' molecules of gas B , each of mass ' 2 m ' are contained in the same vessel which is at constant temperature ' T '. The mean square velocity of $B$ is $V^2$ and mean square of x -component of A is $\omega^2$. The value of $\frac{\omega^2}{\mathrm{~V}^2}$ is
The $\mathrm{p}-\mathrm{V}$ diagram for a fixed mass of an ideal gas undergoing cyclic process is as shown in figure. AB represents isothermal process and CA represents adiabatic process. Which one of the following graphs represents the p-T diagram of this cyclic process?

Two cylinders A and B fitted with piston contain equal amount of an ideal diatomic as at temperature ' T ' K . The piston of cylinder A is free to move while that of B is held fixed. The same amount of heat is given to the gas in each cylinder. If the rise temperature of the gas in A is ' $\mathrm{dT}_{\mathrm{A}}$ ', then the rise in temperature of the gas in cylinder B is $\left(\gamma=\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}\right)$
A metal rod having coefficient of linear expansion $2 \times 10^{-5} /^{\circ} \mathrm{C}$ is 0.75 m long at $45^{\circ} \mathrm{C}$. When the temperature rises to $65^{\circ} \mathrm{C}$, the increase in length of the rod will be
The ratio of the velocity of sound in hydrogen gas $\left(\gamma=\frac{7}{5}\right)$ to that in helium gas $\left(\gamma=\frac{5}{3}\right)$ at the same temperature is
Two spheres $S_1$ and $S_2$ have same radii but temperatures $T_1$ and $T_2$ respectively. Their emissive power is same and emissivity in the ratio 1:4. Then the ratio $T_1: T_2$ is
Two gases A and B having same initial state ( $\mathrm{P}, \mathrm{V}, \mathrm{n}, \mathrm{T}$ ). Now gas A is compressed to $\frac{\mathrm{V}}{8}$ by isothermal process and other gas B is compressed to $\frac{\mathrm{V}}{8}$ by adiabatic process. The ratio of final pressure of gas $A$ and $B$ is (Both gases are monoatomic, $\gamma=5 / 3$)
Two vessels separately contain two ideal gases A and B at the same temperature, pressure of A being twice that of B . Under such conditions, the density of A is found to be 1.5 times the density of $B$. The ratio of molecular weights of $A$ and $B$ is
An insulated container contains a diatomic gas of molar mass ' m '. The container is moving with velocity ' $V$ ', if it is stopped suddenly, the change in temperature is ( $R=$ gas constant)
Rails of material of steel are laid with gaps to allow for thermal expansion. Each track is 10 m long, when laid at temperature $17^{\circ} \mathrm{C}$. The maximum temperature that can be reached is $45^{\circ} \mathrm{C}$. The gap to be kept between the two segments of railway track is
$$\left(\alpha_{\text {steel }}=1.3 \times 10^{-5} /{ }^{\circ} \mathrm{C}\right)$$
In an adiabatic process for an ideal gas, the relation between the universal gas constant ' $R$ ' and specific heat at constant volume ' $\mathrm{C}_{\mathrm{v}}$ ' is $R=0.4 C_v$. The pressure ' $P$ ' of the gas is proportional to the temperature ' $T$ ', of the gas as $T^k$. The value of constant ' K ' is
The black discs $\mathrm{x}, \mathrm{y}$ and z have radii $1 \mathrm{~m}, 2 \mathrm{~m}$ and 3 m respectively. The wavelengths corresponding to maximum intensity are $200 \mathrm{~nm}, 300 \mathrm{~nm}$ and 400 nm respectively. The relation between emissive power $E_x, E_y$ and $E_z$ is
For a gas, $\frac{\mathrm{R}}{\mathrm{C}_{\mathrm{v}}}=0.4$ where R is the universal gas constant and ' $\mathrm{C}_{\mathrm{V}}$ ' is molar specific heat at constant volume. The gas is made up of molecules which are
In a thermodynamic system ' $\Delta \mathrm{U}$ ' represents the increase in internal energy and ' $W$ ' the work done by the system. Which of the following statement is true?
Rate of radiation by a black body is ' R ' at temperature 'T'. Another body has same area but emissivity is 0.2 and temperature 3T. Its rate of radiation is
A Carnot's cycle operating between $T_H=600 \mathrm{~K}$ and $T_c=300 \mathrm{~K}$ produces 1.5 kJ of mechanical work per cycle. The heat transferred to the engine by the reservoir is
An ordinary body cools from ' $4 \theta^{\prime}$ ' to ' $3 \theta^{\prime}$ ' in ' t ' minutes. The temperature of that body after next 't' minutes is (Assume Newton's law of cooling and room temperature is $\theta$)
A black sphere has radius $R$ whose rate of radiation is E at temperature T . If radius is made half and temperature 4 T , the rate of radiation will be
Ordinary bodies P and Q radiate maximum energy with wavelength difference $3 \mu \mathrm{~m}$. The absolute temperature of body P is four times that of Q. The wavelength at which body Q radiates maximum energy is
The average force applied on the wall of a closed container depends as $\mathrm{T}^{\mathrm{x}}$ where T is the temperature of an ideal gas. The value of $x$ is
Which of the following graphs between pressure and volume correctly show isochoric process?

Initial pressure and volume of a gas are ' P ' and ' $V$ ' respectively. First its volume is expanded to ' 4 V ' by isothermal process and then again its volume is reduced to ' V ' by adiabatic process then its final pressure if $\left(\gamma=\frac{3}{2}\right)$
The P-V diagrams for particular gas of different thermodynamic processes are given by

An ideal gas $(\gamma=1.5)$ is expanded adiabatically. To reduce root mean square velocity of molecules two times, the gas should be expanded
A black body radiates power ' P ' and maximum energy is radiated by it at a wavelength $\lambda_0$. The temperature of the black body is now so changed that it radiates maximum energy at the wavelength $\frac{\lambda_0}{4}$. The power radiated by it at new temperature is
The temperature of a liquid falls from 365 K to 359 K in 3 minutes. The time during which temperature of this liquid falls from 342 K to 338 K is [Let the room temperature be 296 K ]
In an isobaric process of an ideal gas, the ratio of work done by the system (W) during the expansion and the heat exchanged $(\mathrm{Q})$ is $\left(\gamma=\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}\right)$
Three identical metal spheres (of same surface area) have red, black and white colors and they are heated up to same temperature. They are allowed to cool. Arrange them from maximum rate of cooling to minimum rate of cooling
At certain temperature, $\operatorname{rod} \mathrm{A}$ and $\operatorname{rod} \mathrm{B}$ of different materials have lengths $\mathrm{L}_{\mathrm{A}}$ and $\mathrm{L}_{\mathrm{B}}$ respectively. Their coefficients of linear expansion are $\alpha_A$ and $\alpha_B$ respectively. It is observed that the difference between their lengths remains constant at all temperatures. The ratio $\mathrm{L}_{\mathrm{A}}: \mathrm{L}_{\mathrm{B}}$ is given by
The internal energy of a gas will increase when it
A gas is contained in closed vessel. The initial temperature of the gas is $100^{\circ} \mathrm{C}$. If the pressure of the gas is increased by $4 \%$, the increase in the temperature of the gas is
For an ideal gas, in an isobaric process, the ratio of heat supplied ' $Q$ ' to the work done ' $w$ ' by the system is ( $\gamma=$ ratio of specific heat at constant pressure to that at constant volume)
The temperature of a gas is $-80^{\circ} \mathrm{C}$. To what temperature the gas should be heated so that the r.m.s. speed is increased by 2 times?
Two bodies ' X ' and ' Y ' at temperatures ' $\mathrm{T}_1$ ' K and ' $T_2$ ' K respectively have the same dimensions. If their emissive powers are same, the relation between their temperatures is
A lead bullet moving with velocity ' $v$ ' strikes a wall and stops. If $50 \%$ of its energy is converted into heat, then the increase in temperature is ( $s=$ specific heat of lead)
If $C_p$ and $C_v$ are molar specific heats of an ideal gas at constant pressure and volume respectively and ' $\gamma$ ' is $\mathrm{C}_{\mathrm{p}} / \mathrm{C}_{\mathrm{v}}$ then $\mathrm{C}_{\mathrm{p}}=$ ( $\mathrm{R}=$ universal gas constant)
The change in the internal energy of the mass of gas, when the volume changes from ' $V$ ' to ' 2 V ' at constant pressure ' $P$ ' is ( $\gamma$ is the ratio of specific heat of gas at constant pressure to specific heat at constant volume)
A pergect gas of volume 5 litre is compressed isothermally to volume of 1 litre. The r.m.s. speed of the molecules will
A real gas behaves as an ideal gas at
According to the law of equipartition of energy the molar specific heat of a diatomic gas at constant volume where the molecule has one additional vibrational mode is
A carnot engine, whose efficiency is $40 \%$ takes heat from a source maintained at temperature 600 K . It is desired to have an efficiency $60 \%$, then the intake temperature for the same exhaust (sink) temperature should be
Two rods of same length \& material transfer a given amount of heat in 12 s when they are joined end to end. But when they are joined length wise parallel to each other they will transfer same amount of heat in same condition in time
An insulated container contains a monoatomic gas of molar mass ' $m$ '. The container is moving with velocity ' $V$ '. If it is stopped suddenly, the change in temperature is ( $R=$ gas constant)
In an isobaric process of an ideal gas, the ratio of work done by the system to the heat supplied $\left(\frac{W}{Q}\right)$ is
A sphere is at temperature 600 K . In an external environment of 200 K , its cooling rate is ' $R$ ' When the temperature of the sphere falls to 400 K , then cooling rate ' $R$ ' will become
A gas expands in such a way that its pressure and volume satisfy the condition $\mathrm{PV}^2=$ constant. Then the temperature of the gas
The r.m.s. velocity of gas molecules kept at temperature $27^{\circ} \mathrm{C}$ in a vessel is $61 \mathrm{~m} / \mathrm{s}$. Molecular weight of gas is nearly
$$\left[\mathrm{R}=8.31 \frac{\mathrm{~J}}{\mathrm{~mol} \mathrm{~K}}\right]$$
A diatomic gas undergoes adiabatic change. Its pressure P and temperature T are related as $\mathrm{P} \propto \mathrm{T}^{\mathrm{x}}$ where the value of x is
A monoatomic gas is heated at constant pressure. The percentage of total heat used for doing external work is
Two rods, one of copper ( Cu$)$ and the other of iron ( Fe ) having initial lengths $\mathrm{L}_1$ and $\mathrm{L}_2$ respectively are connected together to form a single rod of length $L_1+L_2$. The coefficient of linear expansion of Cu and Fe are $\alpha_c$ and $\alpha_i$ respectively. If the length of each rod increases by the same amount when their temperatures are raised by $t^{\circ} \mathrm{C}$, then ratio of $\frac{L_1-L_2}{L_1+L_2}$ will be
The specific heat of argon at constant pressure and constant volume are $C_p$ and $C_v$ respectively. It's density ' $\rho$ ' at N.T.P. will be $[\mathrm{P}$ and T are pressure and temperature respectively at N.T.P.]
The r.m.s. velocity of hydrogen at S.T.P. is ' $u$ ' $\mathrm{m} / \mathrm{s}$. If the gas is heated at constant pressure till its volume becomes three times, then the final temperature of the gas and the r.m.s. speed are respectively
There are two samples A and B of a certain gas, which are initially at the same temperature and pressure. Both are compressed from volume v to $\frac{\mathrm{v}}{2}$. Sample A is compressed isothermally while sample B is compressed adiabatically. The final pressure of $A$ is
Two rods, one of aluminium and the other of steel, having initial lengths ' $\mathrm{L}_1$ ' and ' $\mathrm{L}_2$ ' are connected together to form a single rod of length $\left(L_1+L_2\right)$. The coefficients of linear expansion of aluminium and steel are ' $\alpha_1$ ' and ' $\alpha_2$ ' respectively. If the length of each rod increases by the same amount, when their temperatures are raised by $\mathrm{t}^{\mathrm{L}} \mathrm{C}$, then the ratio $\frac{L_1}{L_1+L_2}$ will be
Given that ' $x$ ' joule of heat is incident on a body. Out of that, total heat reflected and transmitted is ' $y$ ' joule. The absorption coefficient of body is
A diatomic ideal gas is used in Carnot engine as a working substance. If during the adiabatic expansion part of the cycle, the volume of the gas increases from V to 32 V , the efficiency of the engine is
Two spherical black bodies of radii ' $R_1$ ' and ' $\mathrm{R}_2$ ' and with surface temperature ' $\mathrm{T}_1$ ' and ' $\mathrm{T}_2$ ' respectively radiate the same power. The ratio of ' $R_1$ ' to ' $R_2$ ' will be
Rate of flow of heat through a cylindrical rod is ' $\mathrm{H}_1$ '. The temperature at the ends of the rod are ' $T_1$ ' and ' $T_2$ '. If all the dimensions of the rod become double and the temperature difference remains the same, the rate of flow of heat becomes ' $\mathrm{H}_2$ '. Then
A fixed mass of gas at constant pressure occupies a volume ' V '. The gas undergoes a rise in temperature so that the r.m.s. velocity of the molecules is doubled. The new volume will be
In an isobaric process
The average translational kinetic energy of nitrogen (molar mass 28) molecules at a particular temperature is 0.042 eV . The translational kinetic energy of oxygen molecules (molar mass 32) in eV at double the temperature is
The first operation involved in a carnot cycle is
The temperature at which r.m.s. velocity of hydrogen molecules is 4.5 times that of an oxygen molecule at $47^{\circ} \mathrm{C}$ is (Molecular weight of hydrogen and oxygen molecules are 2 and 32 respectively)
A sample of oxygen gas and a sample of hydrogen gas both have the same mass, same volume and the same pressure. The ratio of their absolute temperature is (Molecular wt. of $\mathrm{O}_2 \& \mathrm{H}_2$ is 32 and 2 respectively)
The P-V graph of an ideal gas, cycle is shown. The adiabatic process is described by the region

Railway track is made of steel segments separated by small gaps to allow for linear expansion. The segment of track is 10 m long when laid at temperature $17^{\circ} \mathrm{C}$. The maximum temperature that can be reached is $45^{\circ} \mathrm{C}$. Increase in length of the segment of railway track is ' $x$ ' $\times 10^{-5} \mathrm{~m}$. The value of ' $x$ ' is $\left(\alpha_{\text {steel }}=\right.$ $\left.1.2 \times 10^{-5} /{ }^{\circ} \mathrm{C}\right)$
At S.T.P., the mean free path of gas molecule is 1500 d , where ' $d$ ' is diameter of molecule. What will be the mean free path at 373 K at constant volume?
One mole of an ideal gas at an initial temperature of ' $T$ ' $K$ does ' $6 R$ ' of work adiabatically. If the ratio of specific heats of this gas at constant pressure and at constant volume is $5 / 3$, the final temperature of gas will be $\left(\mathrm{R}=8.31 \mathrm{~J} \mathrm{~mole}^{-1} \mathrm{~K}^{-1}\right)$
The frequency ' $v_{\mathrm{m}}$ ' corresponding to which the energy emitted by a black body is maximum may vary with the temperature ' $T$ ' of the body as shown by the curves ' A ', ' B ', ' C ' and ' D ' in the figure. Which one of these represents the correct variation?

A metal rod cools at the rate of $$4{ }^{\circ} \mathrm{C} / \mathrm{min}$$ whon its temperature is $$90^{\circ} \mathrm{C}$$ and the rate of $$1{ }^{\circ} \mathrm{C} / \mathrm{m}{\text {in }}$$ when its temperature is $$30^{\circ} \mathrm{C}$$. The temperature of the surrounding is
The molecular mass of a gas having r.m.s. speed four times as that of another gas having molecular mass 32 is
At constant temperature, increasing the pressure of a gas by $$5 \%$$ its volume will decrease by
The temperature of a gas is measure of
An ideal refrigerator has freezer at a temperature of $$-13^{\circ} \mathrm{C}$$. The coefficient of performance of the engine is 5. The temperature of the air (to which heat is rejected) is
The pressure and density of a diatomic gas $$\left(\gamma=\frac{7}{5}\right)$$ changes adiabatically from $$(\mathrm{P}, \rho)$$ to $$\left(\mathrm{P}^{\prime}, \rho^{\prime}\right)$$. If $$\frac{\rho^{\prime}}{\rho}=32$$ then $$\frac{\mathrm{P}^{\prime}}{\mathrm{P}}$$ should be
A sphere and a cube, both of copper have equal volumes and are black. They are allowed to cool at same temperature and in same atmosphere. The ratio of their rate of loss of heat will be
A body is said to be opaque to the radiation if (a, r and t are coefficient of absorption, reflection and transmission respectively)
In a thermodynamic system, $$\Delta U$$ represents the increases in its internal energy and dW is the work done by the system then correct statement out of the following is
The temperature of a gas is $$-68^{\circ} \mathrm{C}$$. To what temperature should it be heated, so that the r.m.s. velocity of the molecules be doubled?
A sphere, a cube and a thin circular plate all made of same material and having the same mass are heated to same temperature of $$200^{\circ} \mathrm{C}$$. When these are left in a room.
The efficiency of a heat engine is '$$\eta$$' and the coefficient of performance of a refrigerator is '$$\beta$$'. Then
A sample of oxygen gas and a sample of hydrogen gas both have the same mass, same volume and the same pressure. The ratio of their absolute temperature is
The internal energy of a monoatomic ideal gas molecule is
A gas at pressure $$p_0$$ is contained in a vessel. If the masses of all the molecules are halved and their velocities are doubled, then the resulting pressure would be equal to
For an adiabatic process, which one of the following is wrong statement?
Which one of the following is based on convection?
A carnot engine operates with source at $$227^{\circ} \mathrm{C}$$ and sink at $$27^{\circ} \mathrm{C}$$. If the source supplies $$50 \mathrm{~kJ}$$ of heat energy, the work done by the engine is
Which one of the following represents correctly the variation of volume (V) of an ideal gas with temperature $$(\mathrm{T})$$ under constant pressure conditions?

$$\mathrm{dQ}$$ is the heat energy supplied to an ideal gas under isochoric conditions. If $$\mathrm{dU}$$ and $$\mathrm{dW}$$ denote the change in internal energy and the work done respectively then
A black body at temperature $$127^{\circ} \mathrm{C}$$ radiates heat at the rate of $$5 \mathrm{~cal} / \mathrm{cm}^2 \mathrm{~s}$$. At a temperature $$927^{\circ} \mathrm{C}$$, its rate of emission in units of $$\mathrm{cal} / \mathrm{cm}^2 \mathrm{~s}$$ will be
A Carnot engine has the same efficiency between (i) $$100 \mathrm{~K}$$ and $$600 \mathrm{~K}$$ and (ii) $$\mathrm{T} \mathrm{K}$$ and $$960 \mathrm{~K}$$. The temperature $$\mathrm{T}$$ in kelvin of the sink is
For an ideal gas the density of the gas is $$\rho_0$$ when temperature and pressure of the gas are $$T_0$$ and $$P_0$$ respectively. When the temperature of the gas is $$2 \mathrm{~T}_0$$, its pressure will be $$3 \mathrm{P}_0$$. The new density will be
The temperature gradient in a rod of length $$75 \mathrm{~cm}$$ is $$40^{\circ} \mathrm{C} / \mathrm{m}$$. If the temperature of cooler end of the rod is $$10^{\circ} \mathrm{C}$$, then the temperature of hotter end is
A black body radiates maximum energy at wavelength '$$\lambda$$' and its emissive power is '$$E$$'. Now due to a change in temperature of that body, it radiates maximum energy at wavelength $$\frac{\lambda}{3}$$. At that temperature emissive power is
For polyatomic gases, the ratio of molar specific heat at constant pressure to constant volume is ( $$\mathrm{f}=$$ degrees of freedom)
Select the WRONG statement from the following. For an isothermal process
Compare the rate of loss of heat from a metal sphere at $$627^{\circ} \mathrm{C}$$ with the rate of loss of heat from the same sphere at $$327^{\circ} \mathrm{C}$$, if the temperature of the surrounding is $$27^{\circ} \mathrm{C}$$. (nearly)
The volume of a metal block increases by $$0.225 \%$$ when its temperature is increased by $$30^{\circ} \mathrm{C}$$. Hence coefficient of linear expansion of the material of metal block is
A monoatomic ideal gas initially at temperature '$$\mathrm{T}_1$$' is enclosed in a cylinder fitted with massless, frictionless piston. By releasing the piston suddenly the gas is allowed to expand to adiabatically to a temperature '$$\mathrm{T}_2$$'. If '$$\mathrm{L}_1$$' and '$$\mathrm{L}_2$$' are the lengths of the gas columns before and after expansion respectively, then $$\frac{\mathrm{T}_2}{\mathrm{~T}_1}$$ is
Let $$\gamma_1$$ be the ratio of molar specific heat at constant pressure and molar specific heat at constant volume of a monoatomic gas and $$\gamma_2$$ be the similar ratio of diatomic gas. Considering the diatomic gas molecule as a rigid rotator, the ratio $$\frac{\gamma_2}{\gamma_1}$$ is
The molar specific heat of an ideal gas at constant pressure and constant volume is $$\mathrm{C}_{\mathrm{p}}$$ and $$\mathrm{C}_{\mathrm{v}}$$ respectively. If $$\mathrm{R}$$ is universal gas constant and $$\gamma=\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}$$ then $$\mathrm{C}_{\mathrm{v}}=$$
A composite slab consists of two materials having coefficient of thermal conductivity $$\mathrm{K}$$ and $$2 \mathrm{~K}$$, thickness $$\mathrm{x}$$ and $$4 \mathrm{x}$$ respectively. The temperature of the two outer surfaces of a composite slab are $$\mathrm{T}_2$$ and $$\mathrm{T}_1\left(\mathrm{~T}_2 > \mathrm{T}_1\right)$$. The rate of heat transfer through the slab in a steady state is $$\left[\frac{\mathrm{A}\left(\mathrm{T}_2-\mathrm{T}_1\right) \mathrm{K}}{\mathrm{x}}\right] \cdot \mathrm{f}$$ where '$$\mathrm{f}$$' is equal to
A black sphere has radius '$$R$$' whose rate of radiation is '$$E$$' at temperature '$$T$$'. If radius is made $$R / 3$$ and temperature '$$3 T$$', the rate of radiation will be
A gas at normal temperature is suddenly compressed to one-fourth of its original volume. If $$\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}=\gamma=1.5$$, then the increase in its temperature is
About black body radiation, which of the following is the wrong statement?
For a gas, $$\frac{\mathrm{R}}{\mathrm{C}_{\mathrm{v}}}=0 \cdot 4$$, where $$\mathrm{R}$$ is universal gas constant and $$\mathrm{C}_{\mathrm{v}}$$ is molar specific heat at constant volume. The gas is made up of molecules which are
Two bodies $$\mathrm{A}$$ and $$\mathrm{B}$$ at temperatures '$$\mathrm{T}_1$$' $$\mathrm{K}$$ and '$$\mathrm{T}_2$$' $$\mathrm{K}$$ respectively have the same dimensions. Their emissivities are in the ratio $$1: 3$$. If they radiate the same amount of heat per unit area per unit time, then the ratio of their temperatures $$\left(\mathrm{T}_1: \mathrm{T}_2\right)$$ is
If temperature of gas molecules is raised from $$127^{\circ} \mathrm{C}$$ to $$527^{\circ} \mathrm{C}$$, the ratio of r.m.s. speed of the molecules is respectively
According to Boyle's law, the product PV remains constant. The unit of $$\mathrm{PV}$$ is same as that of
The difference in length between two rods $$\mathrm{A}$$ and $$\mathrm{B}$$ is $$60 \mathrm{~cm}$$ at all temperatures. If $$\alpha_{\mathrm{A}}=18 \times 10^{-6} /{ }^{\circ} \mathrm{C}$$ and $$\beta_{\mathrm{B}}=27 \times 10^{-6} /{ }^{\circ} \mathrm{C}$$, the lengths of the two rods are
An ideal gas expands adiabatically. $$(\gamma=1 \cdot 5)$$ To reduce the r.m.s. velocity of the molecules 3 times, the gas has to be expanded
Two spherical black bodies of radii '$$r_1$$' and '$$r_2$$' at temperature '$$\mathrm{T}_1$$' and '$$\mathrm{T}_2$$' respectively radiate power in the ratio $$1: 2$$ Then $$r_1: r_2$$ is
The rate of flow of heat through a metal rod with temperature difference $$40^{\circ} \mathrm{C}$$ is $$1600 \mathrm{~cal} / \mathrm{s}$$. The thermal resistance of metal rod in $${ }^{\circ} \mathrm{C} \mathrm{s} / \mathrm{cal}$$ is
If the temperature of a hot body is increased by $$50 \%$$, then the increase in the quantity of emitted heat radiation will be approximately
A monoatomic gas at pressure '$$\mathrm{P}$$', having volume '$$\mathrm{V}$$' expands isothermally to a volume '$$2 \mathrm{~V}$$' and then adiabatically to a volume '$$16 \mathrm{~V}$$'. The final pressure of the gas is (Take $$\gamma=5 / 3$$ )
A diatomic gas $$\left(\gamma=\frac{7}{5}\right)$$ is compressed adiabatically to volume $$\frac{V_i}{32}$$ where $$V_i$$ is its initial volume. The initial temperature of the gas is $$T_i$$ in Kelvin and the final temperature is '$$x T_i$$'. The value of '$$x$$' is
If a gas is compressed isothermally then the r.m.s. velocity of the molecules
A black body radiates maximum energy at wavelength '$$\lambda$$' and its emissive power is 'E' Now due to change in temperature of that body, it radiates maximum energy at wavelength $$\frac{2 \lambda}{3}$$. At that temperature emissive power is
Which of the following graphs between pressure (P) and volume (V) correctly shows isochoric changes?

A metal rod $$2 \mathrm{~m}$$ long increases in length by $$1.6 \mathrm{~mm}$$, when heated from $$0^{\circ} \mathrm{C}$$ to $$60^{\circ} \mathrm{C}$$. The coefficient of linear expansion of metal rod is
We have a jar filled with gas characterized by parameters $$\mathrm{P}, \mathrm{V}, \mathrm{T}$$ and another jar B filled with gas having parameters $$2 \mathrm{P}, \frac{\mathrm{V}}{4}, 2 \mathrm{~T}$$, where symbols have their usual meaning. The ratio of number of molecules in jar A to those in jar B is
An insulated container contains a monoatomic gas of molar mass '$$\mathrm{m}$$'. The container is moving with velocity '$$\mathrm{V}$$'. If it is stopped suddenly, the change in temperature of a gas is [R is gas constant]
In a vessel, the ideal gas is at a pressure $$\mathrm{P}$$. If the mass of all the molecules is halved and their speed is doubled, then resultant pressure of the gas will be
The average force applied on the walls of a closed container depends on $$T^x$$ where $$T$$ is the temperature of an ideal gas. The value of '$$x$$' is
A black body radiates maximum energy at wavelength '$$\lambda$$' and its emissive power is $$\mathrm{E}$$. Now due to change in temperature of that body, it radiates maximum energy at wavelength $$\frac{2 \lambda}{3}$$. At that temperature emissive power is
A Carnot engine with efficiency $$50 \%$$ takes heat from a source at $$600 \mathrm{~K}$$. To increase the efficiency to $$70 \%$$, keeping the temperature of the sink same, the new temperature of the source will be
A piece of metal at $$850 \mathrm{~K}$$ is dropped in to $$1 \mathrm{~kg}$$ water at $$300 \mathrm{~K}$$. If the equilibrium temperature of water is $$350 \mathrm{~K}$$ then the heat capacity of the metal, expressed in $$\mathrm{JK}^{-1}$$ is $$(1 \mathrm{~cal}=4.2 \mathrm{~J})$$
Heat energy is incident on the surface at the rate of X J/min . If '$$a$$' and '$$r$$' represent coefficient of absorption and reflection respectively then the heat energy transmitted by the surface in '$$t$$' minutes is
A sample of gas at temperature $$T$$ is adiabatically expanded to double its volume. The work done by the gas in the process is $$\left(\frac{\mathrm{C}_{\mathrm{P}}}{\mathrm{C}_{\mathrm{V}}}=\gamma=\frac{3}{2}\right) \quad(\mathrm{R}=$$ gas constant $$)$$
An ideal gas in a container of volume 500 c.c. is at a pressure of $$2 \times 10^{+5} \mathrm{~N} / \mathrm{m}^2$$. The average kinetic energy of each molecule is $$6 \times 10^{-21} \mathrm{~J}$$. The number of gas molecules in the container is
A gas at N.T.P. is suddenly compressed to onefourth of its original volume. If $$\gamma=1.5$$, then the final pressure is
A gas is compressed at a constant pressure of $$50 \mathrm{~N} / \mathrm{m}^2$$ from a volume of $$10 \mathrm{~m}^3$$ to a volume of $$4 \mathrm{~m}^3$$. Energy of $$100 \mathrm{~J}$$ is then added to the gas by heating. Its internal energy is
The pressure exerted by an ideal gas at a particular temperature is directly proportional to
The side of a copper cube is $$1 \mathrm{~m}$$ at $$0^{\circ} \mathrm{C}$$. What will be the change in its volume, when it is heated to $$100^{\circ} \mathrm{C}$$ ? $$\left[\alpha_{\text {copper }}=18 \times 10^{-6} /{ }^{\circ} \mathrm{C}\right]$$
The temperature of an ideal gas is increased from $$27^{\circ} \mathrm{C}$$ to $$927^{\circ} \mathrm{C}$$. The r.m.s. speed of its molecules becomes
A jar '$$\mathrm{P}$$' is filled with gas having pressure, volume and temperature $$\mathrm{P}, \mathrm{V}, \mathrm{T}$$ respectively. Another gas jar $$Q$$ filled with a gas having pressure $$2 \mathrm{P}$$, volume $$\frac{\mathrm{V}}{4}$$ and temperature $$2 \mathrm{~T}$$. The ratio of the number of molecules in jar $$\mathrm{P}$$ to those in jar $$Q$$ is
For a gas having '$$\mathrm{X}$$' degrees of freedom, '$$\gamma$$' is ($$\gamma=$$ ratio of specific heats $$=\mathrm{C_P / C_V}$$)
Two uniform brass rods $$A$$ and $$B$$ of length '$$l$$' and '$$2 l$$' and their radii '$$2 r$$' and '$$r$$' respectively are heated to same temperature. The ratio of the increase in the volume of $$\operatorname{rod} \mathrm{A}$$ to that of $$\operatorname{rod} \mathrm{B}$$ is
A gas at N.T.P. is suddenly compressed to $$\left(\frac{1}{4}\right)^{\text {th }}$$ of its original volume. The final pressure in (Given $$\gamma=$$ ratio of sp. heats $$=\frac{3}{2}$$ ) atmosphere is ( $$\mathrm{P}=$$ original pressure)
In a thermodynamic process, there is no exchange of heat between the system and surroundings. Then the thermodynamic process is
According to kinetic theory of gases, which one of the following statements is wrong?
Three discs $$\mathrm{x}, \mathrm{y}$$ and $$\mathrm{z}$$ having radii $$2 \mathrm{~m}, 3 \mathrm{~m}$$ and $$6 \mathrm{~m}$$ respectively are coated on outer surfaces. The wavelength corresponding to maximum intensity are $$300 \mathrm{~nm}, 400 \mathrm{~nm}$$ and $$500 \mathrm{~nm}$$ respectively. If $$\mathrm{P}_{\mathrm{x}}, \mathrm{P}_{\mathrm{y}}$$ and $$\mathrm{P}_{\mathrm{z}}$$ are power radiated by them respectively then
When the rms velocity of a gas is denoted by '$$v$$', which one of the following relations is true?
($$\mathrm{T}=$$ Absolute temperature of the gas.)
A monoatomic gas $$\left(\gamma=\frac{5}{3}\right)$$ initially at $$27^{\circ} \mathrm{C}$$ having volume '$$\mathrm{V}$$' is suddenly compressed to one-eighth of its original volume $$\left(\frac{\mathrm{V}}{8}\right)$$. After the compression its temperature becomes
Two monatomic ideal gases A and B of molecular masses '$$m_1$$' and '$$m_2$$' respectively are enclosed in separate containers kept at the same temperature. The ratio of the speed of sound in gas A to that in gas B is given by
The thermodynamic process in which no work is done on or by the gas is
Heat given to a body, which raises its temperature by 1ºC is known as
Which one of the following is NOT a correct expression for an ideal gas?
[$$\mathrm{C_p}=$$ Molar specific heat of a gas at constant pressure,
$$\mathrm{C_v}=$$ Molar specific heat of a gas at constant volume,
$$\mathrm{Y}=$$ Ratio of two specific heats of a gas,
$$\mathrm{R}=$$ Universal gas constant]
The molecular masses of helium and oxygen are 4 and 32 respectively. The ratio of r.m.s. speed of helium at 327$$^\circ$$ to r.m.s. speed of oxygen at 27$$^\circ$$ will be
Which one of the following p-V diagram is correct for an isochoric process:

Assume that for solar radiation, surface temperature of the sun is $$6000 \mathrm{~K}$$. If Wien's constant 'b' is $$2.897 \times 10^{-3} \mathrm{~mK}$$, the value of maximum wavelength will be
A metal sphere cools at the rate of $$1.5^{\circ} \mathrm{C} / \mathrm{min}$$ when its temperature is $$80^{\circ} \mathrm{C}$$. At what rate will it cool when its temperature falls to $$50^{\circ} \mathrm{C}$$. [Temperature of surrounding is $$30^{\circ} \mathrm{C}$$]
A monoatomic gas is suddenly compressed to $$(1 / 8)^{\text {th }}$$ of its initial volume adiabatically. The ratio of the final pressure to initial pressure of the gas is $$(\gamma=5 / 3)$$
A monoatomic ideal gas initially at temperature $$\mathrm{T}_1$$ is enclosed in a cylinder fitted with 8 frictionless piston. The gas is allowed to expand adiabatically to a temperature $$\mathrm{T}_2$$ by releasing the piston suddenly. $$\mathrm{L}_1$$ and $$\mathrm{L}_2$$ are the lengths of the gas columns before and after the expansion respectively. Then $$\frac{\mathrm{T}_2}{\mathrm{~T}_1}$$ is
For a monoatomic gas, the work done at constant pressure is '$$\mathrm{W}$$' The heat supplied at constant volume for the same rise in temperature of the gas is
$$[\gamma=\frac{C_p}{C_v}=\frac{5}{2}$$ for monoatomic gas]
An ideal gas with pressure $$\mathrm{P}$$, volume $$\mathrm{V}$$ and temperature $$\mathrm{T}$$ is expanded isothermally to a volume $$2 \mathrm{~V}$$ and a final pressure $$\mathrm{P}_{\mathrm{i}}$$. The same gas is expanded adiabatically to a volume $$2 \mathrm{~V}$$, the final pressure is $$\mathrm{P}_{\mathrm{a}}$$. In terms of the ratio of the two specific heats for the gas '$$\gamma$$', the ratio $$\frac{P_i}{P_a}$$ is
At what temperature does the average translational kinetic energy of a molecule in a gas becomes equal to kinetic energy of an electron accelerated from rest through potential difference of 'V' volt?
($$\mathrm{N}=$$ number of molecules, $$\mathrm{R}=$$ gas constant, $$\mathrm{c}=$$ electronic charge)
The temperature difference between two sides of an iron plate, $$1.8 \mathrm{~cm}$$ thick is $$9^{\circ} \mathrm{C}$$. Heat is transmitted through the plate $$10 \mathrm{k} \mathrm{cal} / \mathrm{sm}^2$$ at steady state. The thermal conductivity of iron is
Internal energy of $$n_1$$ moles of hydrogen at temperature '$$T$$' is equal to internal energy of '$$n_2$$' moles of helium at temperature $$2 T$$, then the ratio $$\mathrm{n}_1: \mathrm{n}_2$$ is
[Degree of freedom of $$\mathrm{He}=3$$, Degree of freedom of $$\mathrm{H}_2=5$$]
For an ideal gas, $$R=\frac{2}{3} C_v$$. This suggests that the gas consists of molecules, which are [$$\mathrm{R}=$$ universal gas constant]
The rms speed of a gas molecule is '$$\mathrm{V}$$' at pressure '$$\mathrm{P}$$'. If the pressure is increased by two times, then the rms speed of the gas molecule at the same temperature will be
Equal volumes of two gases, having their densíties in the ratio of $$1: 16$$ exert equal pressures on the walls of two containers. The ratio of their rms speads ($$\mathrm{C}_1: \mathrm{C}_2)$$ is
A cylindrical rod has temperatures '$$T_1$$' and '$$T_2$$' at its ends. The rate of flow of heat is '$$Q_1$$' cal $$\mathrm{s}^{-1}$$. If length and radius of the rod are doubled keeping temperature constant, then the rate of flow of heat '$$\mathrm{Q}_2$$' will be
The initial pressure and volume of a gas is '$$\mathrm{P}$$' and '$$\mathrm{V}$$' respectively. First by isothermal process gas is expanded to volume '$$9 \mathrm{~V}$$' and then by adiabatic process its volume is compressed to '$$\mathrm{V}$$' then its final pressure is (Ratio of specific heat at constant pressure to constant volume $$=\frac{3}{2}$$)
If $$\mathrm{m}$$' represents the mass of each molecules of a gas and $$\mathrm{T}$$' its absolute temperature then the root mean square speed of the gas molecule is proportional to
An ideal gas at pressure '$$p$$' is adiabatically compressed so that its density becomes twice that of the initial. If $$\gamma=\frac{c_p}{c_v}=\frac{7}{5}$$, then final pressure of the gas is
Which one of the following statements is wrong for an isobaric process?
For a perfectly black body, coefficient of emission is
Two rods of different metals have coefficients of linear expansion '$$\alpha_1$$' and '$$\alpha_2$$' respectvely. Their respective lengths are '$$\mathrm{L}_1$$' and '$$\mathrm{L}_2$$'. At all temperatures ($$\mathrm{L}_2-\mathrm{L}_1$$) is same. The correct relation is
The temperature of a black body is increased by $$50 \%$$, then the percentage increase in the rate of radiation by the body is approximated
The emissive power of sphere of area $$0.04 \mathrm{~m}^2$$ is $$0.7 \mathrm{~k} \mathrm{~cal} \mathrm{~s}^{-1} \mathrm{~m}^{-2}$$. The amount of heat radiated in 20 second is
The rate of flow of heat through a copper rod with temperature difference $$28^{\circ} \mathrm{C}$$ is $$1400 \mathrm{~cal} \mathrm{~s}^{-1}$$. The thermal resistance of copper rod will be
The change in internal energy of the mass of a gas, when the volume changes from '$$\mathrm{V}$$' to '$$2 \mathrm{~V}$$' at constant pressure 'P' is ($$\gamma=$$ Ratio of Cp to Cv)
If the pressure of an ideal gas is decreased by $$10 \%$$ isothermally, then its volume will
An ideal gas having molar mass '$$\mathrm{M}_0$$', has r.m.s. velocity 'V' at temperature 'T'. Then
An ideal gas at $$27^{\circ} \mathrm{C}$$ is compressed adiabatically to $$(8 / 27)$$ of its original volume. If ratio of specific heats, $$\gamma=5 / 3$$ then the rise in temperature of the gas is
The translational kinetic energy of the molecules of a gas at absolute temperature (T) can be doubled
A polyatomic gas $$(\gamma=4 / 3)$$ is compressed to $$\left(\frac{1}{8}\right)^{\text {th }}$$ of its volume adiabatically. If its initial pressure is $$\mathrm{P}_0$$, its new pressure will be
If the temperature of the sun is doubled, the rate of energy received by the earth will be increased by a factor
Which of the following statements is true?
($$\Delta \mathrm{U}=$$ increase in internal energy, $$\mathrm{dW}=$$ work done by the system)
Let '$$\mathrm{W}_1$$' be the work done in blowing a soap bubble of radius '$$r$$' from soap solution at room temperature. The soap solution is now heated and second soap bubble of radius '$$2 r$$' is blown from the heated soap solution. If '$$W_2$$' is the work done in forming this bubble then
A cylindrical rod is having temperatures $$\theta_1$$ and $$\theta_2$$ at its ends. The rate of heat flow is '$$Q$$' $$\mathrm{J}{\mathrm{s}}^{-1}$$. All the linear dimensions of the rod are doubled by keeping the temperatures constant. What is the new rate of flow of heat?
For a gas molecule with 6 degrees of freedom, which one of the following relation between gas constant '$$\mathrm{R}$$' and molar specific heat '$$\mathrm{C}_{\mathrm{v}}$$' is correct?
What is the ratio of the velocity of sound in hydrogen $$\left(\gamma=\frac{7}{5}\right)$$ to that in helium $$\left(\gamma=\frac{5}{3}\right)$$ at the same temperature? (Molecular weight of hydrogen and helium is 2 and 4 respectively.)
Equal volumes of two gases are kept in different containers having densities in the ratio 1 : 16. They exert equal pressures on the wall of their respective containers. Then the ratio of their r.m.s. velocities is
In thermodynamics, for an isochoric process, which one of the following statement is INCORRECT?
If '$$\mathrm{E}$$' is the kinetic energy per mole of an ideal gas and '$$\mathrm{T}$$' is the absolute temperature, then the universal gas constant is given as
Two rods of same length and material are joined end to end. They transfer heat in 8 second. When they are joined in parallel they transfer same amount of heat in same conditions in time
The molar specific heats of an ideal gas at constant pressure and volume are denoted by '$$\mathrm{C}_{\mathrm{p}}$$' and '$$C_v$$' respectively. If $$\gamma=\frac{C_p}{C_v}$$ and '$$R$$' is universal gas constant, then $$C_v$$ is equal to
The temperature difference bewtween two sides of metal plate, $$3 \mathrm{~cm}$$ thick is $$15^{\circ} \mathrm{C}$$. Heat is transmitted through plate at the rate of $$900 \mathrm{~kcal}$$ per minute per $$\mathrm{m}^2$$ at steady state. The thermal conductivity of metal is
A black body has maximum wavelength '$$\lambda_{\mathrm{m}}$$' at temperature $$2000 \mathrm{~K}$$. Its corresponding wavelength at temperature $$3000 \mathrm{~K}$$ will be
A monoatomic gas at pressure '$$\mathrm{P}$$' having volume '$$\mathrm{V}$$' expands isothermally to a volume $$2 \mathrm{~V}$$ and then adiabatically to a volume $$16 \mathrm{~V}$$. The final pressure of the gas is $$\left(\gamma=\frac{5}{3}\right)$$
A black reactangular surface of area '$$a$$' emits energy '$$\mathrm{E}$$' per second at $$27^{\circ} \mathrm{C}$$. If length and breadth is reduced to $$\left(\frac{1}{3}\right)^{\text {rd }}$$ of initial value and temperature is raised to $$327^{\circ} \mathrm{C}$$ then energy emitted per second becomes
Find the value of $$-$$197$$^\circ$$C temperature in Kelvin.
Which one of the following equations specifies an isobaric process? $$[Q=$$ heat supplied $$\Delta P, \Delta V$$ and $$\Delta T$$ are change in pressure, volume and temperature respectively]
A perfect gas of volume 10 litre n compressed isothermally to a volume of 1 litre. The rms speed of the molecules will
The relation obeyed by a perfect gas during an adiabatic process is $$\mathrm{PV}^{3 / 2}$$. The initial temperature of the gas is '$$\mathrm{T}$$'. When the gas is compressed to half of its Initial volume, the final temperature of the gas is
A black rectangular surface of area '$$\mathrm{A}$$' emits energy '$$\mathrm{E}$$' per second at $$27^{\circ} \mathrm{C}$$. If length and breadth is reduced to $$(1 / 3)^{\text {rd }}$$ of its initial value and temperature is raised to $$327^{\circ} \mathrm{C}$$ then energy emitted per second becomes
A monoatomic gas is suddenly compressed to (1/8)th of its initial volume adiabatically. The ratio of the final pressure to initial pressure of the gas is ($$\gamma=5/3$$)
A conducting rod of length $$1 \mathrm{~m}$$ has area of cross-section $$10^{-3} \mathrm{~m}^2$$. One end is immersed in baiting water $$\left(100^{\circ} \mathrm{C}\right)$$ and the other end in Ice $$\left(0^{\circ} \mathrm{C}\right)$$. If coefficient of thermal conductivity of $$\mathrm{rod}$$ is $$96 \mathrm{~cal} / \mathrm{sm}^{\circ} \mathrm{C}$$ and latent heat for ice is $$8 \times 10^{-4} \mathrm{cal} / \mathrm{kg}$$ then the amount of ice which will melt in one minute is
Two stars 'P' and 'Q' emit yellow and blue light respectively. The relation between their temperatures $$\left(\mathrm{T}_{\mathrm{P}}\right.$$ and $$\left.\mathrm{T}_{\mathrm{Q}}\right)$$ is
A perfectly black body emits a radiation at temperature 'T$$_1$$' K. If it is to radiate at 16 times this power, its temperature 'T$$_2$$' K should be
One mole of an ideal gas expands adiabatically at constant pressure such that its temperature $$T \propto {1 \over {\sqrt V }}$$. The value of $$\gamma$$ for the gas is ($$\gamma = {{{C_p}} \over {{C_v}}},V = $$ Volume of the gas)
On an imaginary linear scale of temperature (called 'W' scale) the freezing and boiling points of water are 39$$^\circ$$ W and 239$$^\circ$$ W respectively. The temperature on the new scale corresponding to 39$$^\circ$$C temperature on Celsius scale will be
Specific heats of an ideal gas at constant pressure and volume are denoted by $$\mathrm{C}_{\mathrm{p}}$$ and $$\mathrm{C}_{\mathrm{v}}$$ respectively. If $$\gamma=\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}$$ and $$\mathrm{R}$$ it's the universal gas constant then $$\mathrm{C}_{\mathrm{v}}$$ is equal to
For a monoatomic gas, work done at constant pressure is W. The heat supplied at constant volume for the same rise in temperature of the gas is
The root mean square velocity of molecules of a gas is $200 \mathrm{~m} / \mathrm{s}$. What will be the root mean square velocity of the molecules, if the molecular weight is doubled and the absolute temperature is halved?
Two spherical black bodies of radius $$r_1$$ and $$r_2$$ with surface temperature $$T_1$$ and $$T_2$$ respectively, radiate same power, then $$r_1: r_2$$ is
A diatomic gas undergoes adiabatic change. Its pressure $$p$$ and temperature $$T$$ are related as $$p \propto T^x$$, where $$x$$ is
For a gas, $$\frac{R}{C_V}=0.4$$, where $$R$$ is universal gas constant and $$C_V$$ is the molar specific heat at constant volume. The gas is made up of molecules, which are
A monoatomic gas of pressure $$p$$ having volume $$V$$ expands isothermally to a volume $$2V$$ and then adiabatically to a volume $$16 \mathrm{~V}$$. The final pressure of the gas is (ratio of specific heats $$=\frac{5}{3}$$
The SI unit and dimensions of Stefan's constant $\sigma$ in case of Stefan's law of radiation is
The rms speed of oxygen molecule in a gas is $u$, If the temperature is doubled and the molecules dissociates into two atoms, the rms speed will be
The equation of state for 2 g of oxygen at a pressure ' $P$ ' and temperature ' $T$, when occupying a volume ' $V$ ' will be
The maximum wavelength of radiation emitted by a star is 289.8 nm . Then intensity of radiation for the star is (Given : Stefan's constant $=5.67 \times 10^{-8} \mathrm{Wm}^{-2} \mathrm{~K}^{-4}$, Wien's constant, $b=2898 \mu \mathrm{mK}$ )
If ' $C_P$ ' and ' $C_V$ ' are molar specific heats of an ideal gas at constant pressure and volume respectively. If ' $\lambda$ ' is the ratio of two specific heats and ' $R$ ' is universal gas constant then ' $C_p$ ' is equal to
A clock pendulum having coefficient of linear expansion. $\alpha=9 \times 10^{-7} /{ }^{\circ} \mathrm{C}^{-1}$ has a period of 0.5 s at $20^{\circ} \mathrm{C}$. If the clock is used in a climate, where the temperature is $30^{\circ} \mathrm{C}$, how much time does the clock lose in each oscillation? ( $g=$ constant)
If $\alpha$ is the coefficient of performance of a refrigerator and ' $Q$ ' is heat released to the hot reservoir, then the heat extracted from the cold reservoir ' $Q_2$ ' is