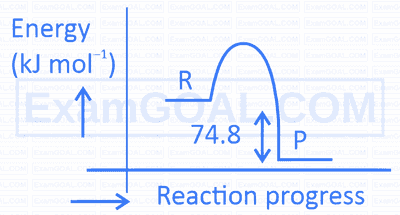
$\mathrm{C}(\mathrm{s})+2 \mathrm{H}_2(\mathrm{~g}) \rightarrow \mathrm{CH}_4(\mathrm{~g}) ; \Delta \mathrm{H}=-74.8 \mathrm{~kJ} \mathrm{~mol}^{-1}$. Which of the following diagrams gives an accurate representation of the above reaction? [ $\mathrm{R} \rightarrow$ reactants; $\mathrm{P} \rightarrow$ products]

Choose the correct statement for the work done in the expansion and heat absorbed or released when 5 litres of an ideal gas at 10 atmospheric pressure isothermally expands into vacuum until volume is 15 litres :

For an endothermic reaction:
(A) $$\mathrm{q}_{\mathrm{p}}$$ is negative.
(B) $$\Delta_{\mathrm{r}} \mathrm{H}$$ is positive.
(C) $$\Delta_r \mathrm{H}$$ is negative.
(D) $$\mathrm{q}_{\mathrm{p}}$$ is positive.
Choose the correct answer from the options given below:

For the following reaction at $$300 \mathrm{~K}$$
$$\mathrm{A}_2(\mathrm{~g})+3 \mathrm{~B}_2(\mathrm{~g}) \rightarrow 2 \mathrm{AB}_3(\mathrm{~g})$$
the enthalpy change is $$+15 \mathrm{~kJ}$$, then the internal energy change is :