Two statements, one Assertion (A) and the other Reason (R) are given. Choose the correct option.
Assertion: 2-aminoethanoic acid and p-aminobenzene sulphonic acid can exist as Zwitter ions while p-aminobenzoic acid cannot.
Reason: When the acid group is a relatively strong proton donor and the $-\mathrm{NH}_2$ group is sufficiently basic it can accept a $\mathrm{H}^{+}$ion from the acid group to form the dipolar ion.
Two statements, One Assertion [ A ] and the other Reason [ R ] are given. Identify the correct option
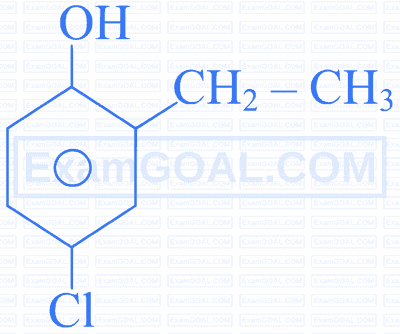
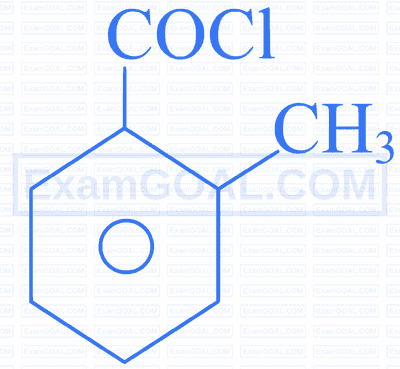
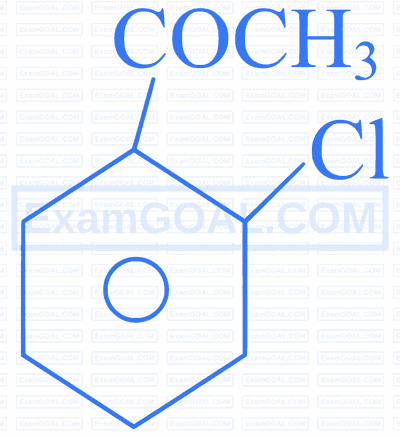
Assertion $[A]$ : The decreasing order of the acidic character of the following is $B>D>A>C$

Reason $[\mathbf{R}]$ : Fluorine has larger -I effect than Cl and Br .
Identify the final product formed when Benzamide undergoes the following reactions: