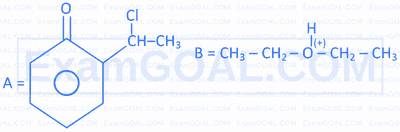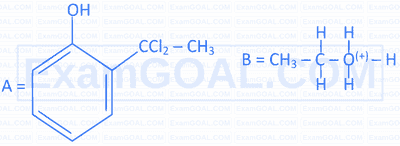1
COMEDK 2025 Evening Shift
MCQ (Single Correct Answer)
+1
-0
Choose the incorrect statement.
2
COMEDK 2025 Evening Shift
MCQ (Single Correct Answer)
+1
-0
Identify $[\mathrm{X}]$ used in the given reaction.
$[\mathrm{X}]+$ Copper $/ 573 \mathrm{~K} \rightarrow[\mathrm{Y}]$
$[\mathrm{Y}] \xrightarrow[\text { (ii) } \mathrm{H}_2 \mathrm{O} / \mathrm{H}^{+}]{\text {(i) } \mathrm{CH}_3 \mathrm{MgBr}}$ Tert.butyl alcohol. (major product)
3
COMEDK 2025 Evening Shift
MCQ (Single Correct Answer)
+1
-0
What are the intermediates formed during the reactions $A$ and $B$ ?
A. Reimer-Tiemann reaction.
B. Dehydration of alcohols in the slow rate determining step.
4
COMEDK 2025 Afternoon Shift
MCQ (Single Correct Answer)
+1
-0
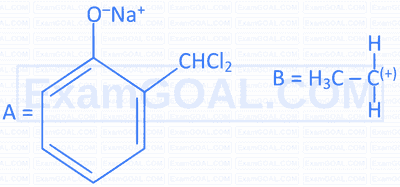
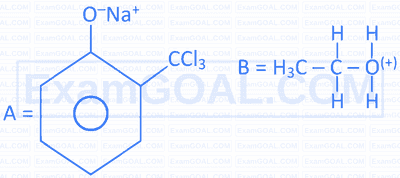
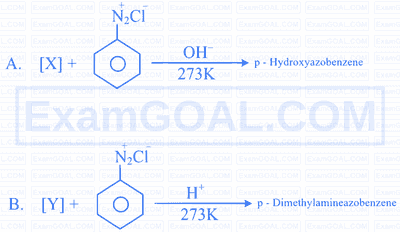
Complete the following 2 reactions A \& B by choosing appropriate reactants $[\mathrm{X}] \&[\mathrm{Y}]$.
Questions Asked from Alcohol, Phenols and Ethers (MCQ (Single Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
COMEDK Subjects
Physics
Mechanics
Units & Measurements Vector Algebra Motion Laws of Motion Circular Motion Work, Energy and Power Center of Mass and Collision Rotational Motion Elasticity Fluid Mechanics Heat and Thermodynamics Gravitation Simple Harmonic Motion Waves
Optics
Electromagnetism
Electrostatics Current Electricity Capacitor Moving Charges and Magnetism Magnetism and Matter Electromagnetic Induction Alternating Current Electromagnetic Waves
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Atomic Structure States of Matter Thermodynamics Chemical Equilibrium Ionic Equilibrium Liquid Solution Redox Reactions Surface Chemistry Solid State Electrochemistry Chemical Kinetics Nuclear Chemistry
Inorganic Chemistry
Periodic Table and Periodicity Chemical Bonding and Molecular Structure Hydrogen and It's Compounds s-Block Elements p-Block Elements d and f Block Elements Metallurgy Coordination Compounds Environmental Chemistry
Organic Chemistry
Mathematics
Algebra
Sets and Relations Logarithms Complex Numbers Quadratic Equations Sequences and Series Permutations and Combinations Probability Binomial Theorem Vector Algebra Three Dimensional Geometry Matrices and Determinants Statistics Mathematical Reasoning Linear Programming
Trigonometry
Trigonometric Ratios & Identities Trigonometric Equations Inverse Trigonometric Functions Properties of Triangles
Coordinate Geometry
Calculus