Alcohol, Phenols and Ethers · Chemistry · COMEDK
MCQ (Single Correct Answer)
Identify $[\mathrm{X}]$ used in the given reaction.
$[\mathrm{X}]+$ Copper $/ 573 \mathrm{~K} \rightarrow[\mathrm{Y}]$
$[\mathrm{Y}] \xrightarrow[\text { (ii) } \mathrm{H}_2 \mathrm{O} / \mathrm{H}^{+}]{\text {(i) } \mathrm{CH}_3 \mathrm{MgBr}}$ Tert.butyl alcohol. (major product)
What are the intermediates formed during the reactions $A$ and $B$ ?
A. Reimer-Tiemann reaction.
B. Dehydration of alcohols in the slow rate determining step.
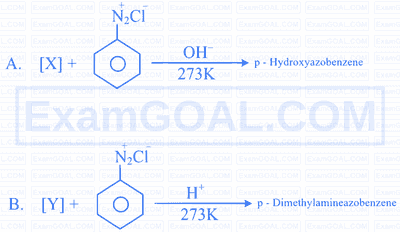
Complete the following 2 reactions A \& B by choosing appropriate reactants $[\mathrm{X}] \&[\mathrm{Y}]$.
Two statements, One Assertion and the other Reason, are given.
Which one of the following is the correct option?
Assertion: The acid strength of 4 compounds in the descending order is p- Nitrophenol > p-Methoxyphenol > Phenol > p-chlorophenol.
Reason: Electron withdrawing groups increase the acid strength while Electron donating groups decrease the acid strength of Phenol and its derivatives.
Which one of the following is the major product formed when the given reaction occurs?

Match the reaction in Column I with the major product formed given in Column II.
| Column I - Reaction/conditions | Column II - Product | ||
|---|---|---|---|
| A. |  |
P. | Pentan-2-ol |
| B. |  |
Q. | 2-Hydroxybenzoic acid. |
| C. |  |
R. | Phenol |
| D. |  |
S. | 2-Methylpropan-1-ol |
Convert Benzene $\rightarrow 3$-Bromophenol by choosing appropriate reagents [(i) to (v)] in the correct sequence.
(i) $\mathrm{NaNO}_2 / \mathrm{HCl}\left(0^{\circ} \mathrm{C}\right)$
(ii) Conc. $\mathrm{HNO}_3 / \mathrm{H}_2 \mathrm{SO}_4$
(iii) $\mathrm{H}_2 \mathrm{O} / 283 \mathrm{~K}$
(iv) $\mathrm{Fe} / \mathrm{HCl}$
(v) $\mathrm{Br} 2 / \mathrm{Fe}$
What is the final product $[\mathrm{Z}]$ formed when the given reactions take place?

Cyclohexanol undergoes a series of reactions as given. Identify compound (iv).
$\mathrm{C}_6 \mathrm{H}_{11} \mathrm{OH}+\mathrm{CrO}_3 \rightarrow(\mathrm{i})+\mathrm{C}_6 \mathrm{H}_5 \mathrm{MgI} \rightarrow(\mathrm{ii})+$ dil. $\mathrm{HCl} \rightarrow(\mathrm{iii})+$ Conc. $\mathrm{H}_3 \mathrm{PO}_4 \rightarrow(\mathrm{iv})$
4 statements are given below. Identify the incorrect statement
A. Phenol has lower $$\mathrm{pK}_{\mathrm{a}}$$ value than $$\mathrm{p}$$-cresol
B. 2-Chlorophenol is more acidic than phenol
C. Ortho and para nitrophenols can be separated by steam distillation since $$\mathrm{p}$$-Nitrophenol is more steam volatile than o-Nitrophenol
D. Phenol on reaction with $$\frac{\mathrm{Cr}_2 \mathrm{O}_7^{2-}}{\mathrm{H}^{+}}$$ yields a conjugated diketone
Two statements, Assertion and Reason are given below. Choose the correct option.
Assertion: n-propyl tert-butyl ether can be readily prepared in the laboratory by Williamson's synthesis.
Reason: The reaction occurs by $$\mathrm{S}_{\mathrm{N}} 1$$ attack of Primary alkoxide on Tert-alkyl halide to give good yield of the product, n-propyl tert-butyl ether.
Compounds $$\mathrm{A}$$ and $$\mathrm{B}$$, having the same molecular formula $$(\mathrm{C}_4 \mathrm{H}_8 \mathrm{O})$$, react separately with $$\mathrm{CH}_3 \mathrm{MgBr}$$, followed by reaction with dil. $$\mathrm{HCl}$$ to form compounds $$\mathrm{X}$$ and $$\mathrm{Y}$$ respectively. Compound $$\mathrm{Y}$$ undergoes acidic dehydration in presence of Conc. $$\mathrm{H}_2 \mathrm{SO}_4$$ much more readily than $$\mathrm{X}$$. Compound $$\mathrm{Y}$$ also reacts with Lucas reagent, much more readily than $$\mathrm{X}$$, with appearance of turbidity. Identify $$\mathrm{X}$$ and $$\mathrm{Y}$$.
The major product '$$\mathrm{X}$$' of the following reaction is

Which of the following tests may be used to distinguish between phenol and cyclohexanol?
Arrange the following compounds in the decreasing order of their acidic strength.
(I) m-cresol
(II) Phenol
(III) m-aminophenol
(IV) m-methoxyphenol
Which of the following compound cannot be prepared by Williamson's synthesis?
An organic compound $$[\mathrm{A}]\left(\mathrm{C}_5 \mathrm{H}_{12} \mathrm{O}\right)$$, on reaction with conc. $$\mathrm{H}_2 \mathrm{SO}_4$$ at $$443 \mathrm{~K}$$ gives $$[\mathrm{B}]$$ as one of the products. Compound [B] undergoes further reaction with $$\mathrm{O}_3$$ and $$\mathrm{Zn} / \mathrm{H}_2 \mathrm{O}$$ to give Propanone and Ethanal as products. Identify compound [A]
Match the compounds given in column I with the corresponding most stable Carbocations formed by each, as given in Column II, when they undergo acidic dehydration in presence of Conc. $$\mathrm{H}_2 \mathrm{SO}_4$$.
| No. | Column I | No. | Column II |
|---|---|---|---|
| A | 2-Methylbutan-1-ol | P | $${(C{H_3})_2}\mathop C\limits^ + - CH{(C{H_3})_2}$$ |
| B | Butan-2-ol | Q | $$C{H_3} - C{H_2} - C{H_2} - \mathop C\limits^ + {H_2}$$ |
| C | 3, 3-Dimethylbutan-2-ol | R | $${(C{H_3})_2}\mathop C\limits^ + - C{H_2} - C{H_3}$$ |
| D | Butan-1-ol | S | $$C{H_3} - \mathop C\limits^ + H - C{H_2} - C{H_3}$$ |

Identify the starting compound from the following data:
$$\mathrm{C}_6 \mathrm{H}_{14} \mathrm{O}(\mathrm{X})$$ on reaction with $$\mathrm{HI}$$ yields a haloalkane (A) and an alcohol (B). Compound (A) on reaction with aqueous $$\mathrm{NaOH}$$ gives an alcohol (C). Compounds (B) and (C) on reaction with $$\mathrm{CrO}_3$$ in anhydrous medium yields Butanone and Ethanal respectively.
Choose the incorrect statement.
Choose the correct order of increasing acidic strength of the following compounds.
The chemical formula of Lucas reagent is
Which of the following is a false statement?
The major product in the given reaction is

Denatured alcohol is