Haloalkanes and Haloarenes · Chemistry · COMEDK
MCQ (Single Correct Answer)
Identify the reagents to be used to complete the given reaction.
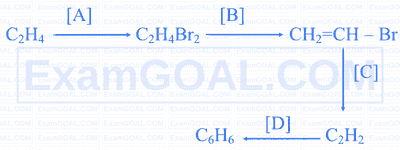
Identify the final product $[Z]$ formed when Chlorobenzene undergoes the given series of reactions:

Both reactions (i) and (ii) give the same compound X as the major product. Identify X
(i). 3-Methylbut-1-ene $+\mathrm{HCl} \rightarrow \mathrm{X}$
(ii). Neopentyl alcohol $+\mathrm{HCl}\left(\right.$ anh. $\left.\mathrm{ZnCl}_2\right) \rightarrow \mathrm{X}$
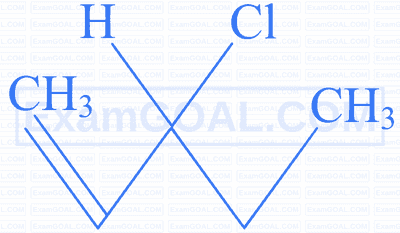
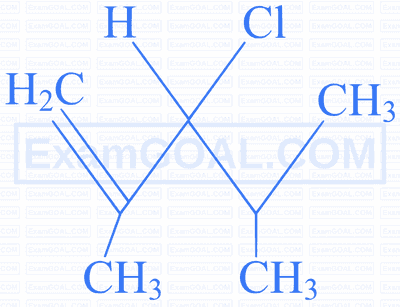
Identify the compound which gives an optically active haloalkane on reaction with $\mathrm{H}_2$ / Ni on heating.
| A | B | C | D |
|---|---|---|---|
 |
 |
 |
 |
Identify the final product formed when Toluene undergoes a series of reactions with reagents given in the order:
(i) $$\mathrm{Cl}_2$$ / Sunlight
(ii) $$\mathrm{H}_2 \mathrm{O} / 373 \mathrm{~K}$$
(iii) Acetophenone / $$\mathrm{OH^-}$$ at $$293 \mathrm{~K}$$
Arrange the following compounds in the increasing order of their reactivity when each of them is reacted with chloroethane / anhydrous AlCl$$_3$$.

Match the names of reactions given in Column I with the appropriate reactions given in Column II.
| No. | Name of reaction | No. | Equations representing reactions |
|---|---|---|---|
| (A) | Sandmeyer | (P) | $$ 2 \mathrm{C}_6 \mathrm{H}_5 \mathrm{Cl}+\mathrm{Na} \text { (dry ether) } \rightarrow \text { Diphenyl. } $$ |
| (B) | Fittig | (Q) | $$ \mathrm{CH}_3-\mathrm{CHBr}-\mathrm{CH}_3+\mathrm{AgF} \rightarrow \mathrm{CH}_3-\mathrm{CHF}-\mathrm{CH}_3+\mathrm{AgBr} $$ |
| (C) | Swarts | (R) | $$ \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{Br}+\mathrm{Nal} \text { ( Acetone) } \rightarrow \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{I}+\mathrm{NaBr} $$ |
| (D) | Finkelstein | (S) | $$ \mathrm{C}_6 \mathrm{H}_5 \mathrm{~N}_2+\mathrm{Cl}-+\mathrm{Cu}_2 \mathrm{Br}_2 / \mathrm{HBr} \rightarrow \mathrm{C}_6 \mathrm{H}_5 \mathrm{Br}+\mathrm{N}_2 $$ |
Arrange the compounds $$\mathrm{A}, \mathrm{B}, \mathrm{C}$$ and $$\mathrm{D}$$ in the increasing order of their reactivity towards $$\mathrm{S_N}1$$ reaction.
$$ \begin{aligned} & \mathrm{A}=\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Cl} \\ & \mathrm{B}=\mathrm{C}_6 \mathrm{H}_5 \mathrm{Cl} \\ & \mathrm{C}=\mathrm{CH}_2=\mathrm{CH}-\mathrm{Cl} \\ & \mathrm{D}=\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{Cl} \end{aligned}$$
Identify the products C, D and F formed in the following sets of reactions.

Arrange the following compounds in the decreasing order of reactivity towards electrophilic substitution reaction.
(I) Chlorobenzene
(II) Nitrobenzene
(III) Benzene
(IV) Isopropylbenzene
Arrange the following in the order of increasing reactivity towards $$\mathrm{SN}_{\mathrm{2}}$$ reactions.
$$\begin{array}{ll} \left(\mathrm{CH}_3\right)_3 \mathrm{CCH}_2 \mathrm{Br}(\mathrm{P}) ; & \\\mathrm{CH}_3\left(\mathrm{CH}_2\right)_3 \mathrm{Br}(\mathrm{Q}) ; &\\ \mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CH}\left(\mathrm{CH}_3\right)-\mathrm{CH}_2 \mathrm{Br}(\mathrm{R}) ; & \\\left(\mathrm{CH}_3\right)_2 \mathrm{CHCH}_2 \mathrm{CH}_2 \mathrm{Br}(\mathrm{S}) \end{array}$$
Choose the incorrect statement from the following.
Consider the following reaction
$$(\mathrm{CH}_3)_2 \mathrm{CHCH}_2 \mathrm{Br}+(\mathrm{CH}_3)_3 \mathrm{CCH}_2 \mathrm{Br} \xrightarrow[\Delta]{\mathrm{Na} \text { /dry ether }} \mathrm{X}+\mathrm{Y}+\mathrm{Z}$$
Identify the product which is not formed?
The major product obtained in the following reaction is :

Which one of the following compounds when reacted with $$\mathrm{NaOH}$$ (aq) undergoes Substitution Nucleophilic Bimolecular reaction with retention of configuration?
$$t$$-butyl chloride preferably undergo hydrolysis by
In Friedal-Crafts alkylation reaction of phenol with chloromethane, the product formed will be

Identify A, B and C.
Identify the compounds A, B, C and D.
$$ \text { 1,2,2,2-tetrachloroethane } \xrightarrow[\Delta]{\mathrm{Zn}}[\mathrm{A}] $$
[A] $$ \xrightarrow[675 \mathrm{~K}]{\text { Fe tube }} $$ [B] $$ \xrightarrow[\text { Conc. } \mathrm{H}_2 \mathrm{SO}_4]{\text { Conc. } \mathrm{HNO}_3} $$ [C] $$ \xrightarrow[\text { Anhydrous } \mathrm{AlCl}_3]{\mathrm{Cl} /}$$ [D]
The reactions taking place with $$\mathrm{2- Phenyl-2-bromopropane}$$ as the starting material is shown below. Identify [A] and [B] formed in the reaction.
$$ \mathrm{C}_6 \mathrm{H}_5-\mathrm{C}\left(\mathrm{CH}_3\right)_2^{-} \mathrm{Br} \xrightarrow[\Delta]{\text { KOH Alcoholic }}[\mathrm{A}] $$
$$ [\mathrm{A}]+\mathrm{HBr} \xrightarrow{\left(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CO})_2 \mathrm{O}\right.}[\mathrm{B}]$$
Which one of the following will undergo Nucleophilic substitution, by $$\mathrm{S}_{\mathrm{N}}{ }^1$$ mechanism, fastest?
Alkyl halides undergoing SN2 reaction do inverse
The reaction $$\mathrm{ArN_2^ + C{l^ - }\buildrel {Cu/HCl} \over \longrightarrow ArCl + {N_2} + CuCl}$$ is called as
Benzene reacts with chlorine in sunlight to give a final product
An alkyl halide reacts with alcoholic ammonia in a sealed tube, the product formed will be