Two statements, One Assertion and the other Reason, are given.
Which one of the following is the correct option?
Assertion: The acid strength of 4 compounds in the descending order is p- Nitrophenol > p-Methoxyphenol > Phenol > p-chlorophenol.
Reason: Electron withdrawing groups increase the acid strength while Electron donating groups decrease the acid strength of Phenol and its derivatives.
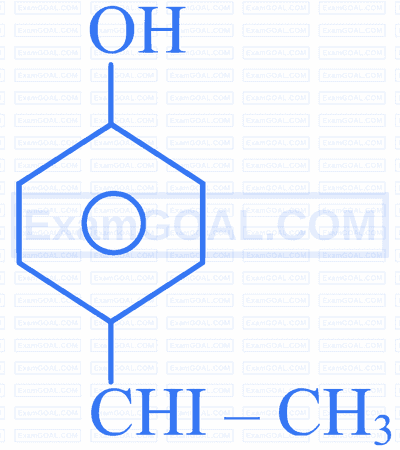
Which one of the following is the major product formed when the given reaction occurs?

Match the reaction in Column I with the major product formed given in Column II.
| Column I - Reaction/conditions | Column II - Product | ||
|---|---|---|---|
| A. |  |
P. | Pentan-2-ol |
| B. |  |
Q. | 2-Hydroxybenzoic acid. |
| C. |  |
R. | Phenol |
| D. |  |
S. | 2-Methylpropan-1-ol |
Convert Benzene $\rightarrow 3$-Bromophenol by choosing appropriate reagents [(i) to (v)] in the correct sequence.
(i) $\mathrm{NaNO}_2 / \mathrm{HCl}\left(0^{\circ} \mathrm{C}\right)$
(ii) Conc. $\mathrm{HNO}_3 / \mathrm{H}_2 \mathrm{SO}_4$
(iii) $\mathrm{H}_2 \mathrm{O} / 283 \mathrm{~K}$
(iv) $\mathrm{Fe} / \mathrm{HCl}$
(v) $\mathrm{Br} 2 / \mathrm{Fe}$