From the following p-V diagram, an ideal gas undergoing a change of state from A to B. Four different processes I, II, III and IV as shown in the figure may lead to same change of state.

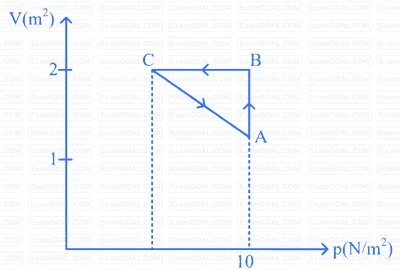
An ideal gas is taken through the cycle A $$\to$$ B $$\to$$ C $$\to$$ A, as shown in the figure. If the net heat supplied to the gas in the cycle is 5 J, the work done by the gas in the process C $$\to$$ A is,
The average kinetic energy of a molecule in air at room temperature of 20$$^\circ$$C
There are two identical containers C$$_1$$ and C$$_2$$ containing to identical gases. Gas in C$$_1$$ is reduced to half of its original volume adiabatically, while the gas in container C$$_2$$ is also reduced to half of its initial volume isothermally. Find the ratio of final pressure in these containers. ($$\gamma$$ be the adiabatic constant).