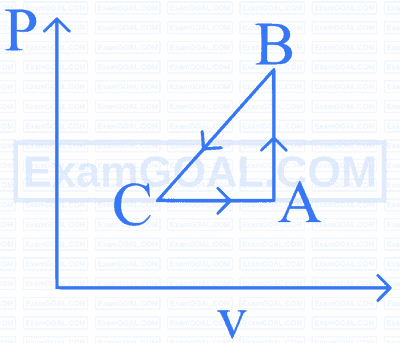
A sample of an ideal gas is taken through the cyclic process ABCA as shown in figure below. It absorbs 60 J of heat during the part AB and rejects 80 J of heat during CA . There is no heat exchanged during the process $\mathrm{BC} . \mathrm{A}$ work of 40 J is done on the gas during the part BC . If the internal energy of the gas at A is 1450 J , then the work done by the gas during the part CA is:
The coefficient of volume expansion of glycerine is $$49 \times 10^{-5} \mathrm{~K}^{-1}$$. The percentage change in its density for a $$50^{\circ} \mathrm{C}$$ rise in temperature is
The latent heat of vaporisation of water is $$2240 \mathrm{~J}$$. If the work done in the process of vaporisation of $$1 \mathrm{~g}$$ is $$168 \mathrm{~J}$$, the increase in internal energy is
Internal energy of $$\mathrm{n}_1$$ moles of hydrogen at temperature T is equal to internal energy of $$\mathrm{m}_2$$ moles of helium at temperature 2T. The ratio $$\frac{n_1}{n_2}$$ is