1
AP EAPCET 2024 - 19th May Evening Shift
MCQ (Single Correct Answer)
+1
-0
Given below are two statements :
Statement I For isothermal irreversible change of an ideal gas, $q=-w=p_{\text {ext }}\left(V_{\text {final }}-V_{\text {initial }}\right)$
Statement II For adiabatic change, $\Delta U=w_{\text {adiabatic }}$
The correct answer is :
2
AP EAPCET 2024 - 19th May Evening Shift
MCQ (Single Correct Answer)
+1
-0
A thermodynamic process $(B \rightarrow E)$ was completed as shown below. The work done is equal to area under the limits 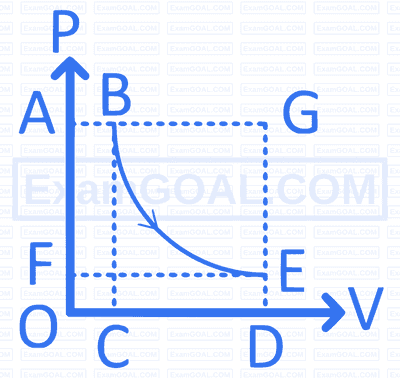

3
AP EAPCET 2024 - 18th May Morning Shift
MCQ (Single Correct Answer)
+1
-0
At 300 K for the reaction. $A \rightarrow P$. The $\Delta S_p$ is $5 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}$, What is the heat absorbed (in kJ $\mathrm{mol}^{-1}$ ) by the system?
4
AP EAPCET 2024 - 18th May Morning Shift
MCQ (Single Correct Answer)
+1
-0
Identify the incorrect statements form the following.
I. $ \Delta S_{\text {pum }}=\left(\Delta S_{\text {nal }}+\Delta S_{\text {um }}\right) $
II. $A(\bar{i} \rightarrow A(\phi)$ : For this process entropy change decreases.
III. Entropy units are $\mathrm{JK} \mathrm{mol}^{-1}$.
Questions Asked from Thermodynamics (MCQ (Single Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
AP EAPCET 2024 - 23th May Morning Shift (1)
AP EAPCET 2024 - 22th May Evening Shift (3)
AP EAPCET 2024 - 22th May Morning Shift (2)
AP EAPCET 2024 - 21th May Evening Shift (2)
AP EAPCET 2024 - 21th May Morning Shift (4)
AP EAPCET 2024 - 20th May Evening Shift (3)
AP EAPCET 2024 - 20th May Morning Shift (2)
AP EAPCET 2024 - 19th May Evening Shift (2)
AP EAPCET 2024 - 18th May Morning Shift (2)
AP EAPCET 2022 - 5th July Morning Shift (2)
AP EAPCET 2022 - 4th July Evening Shift (4)
AP EAPCET 2022 - 4th July Morning Shift (2)
AP EAPCET 2021 - 20th August Morning Shift (1)
AP EAPCET 2021 - 19th August Evening Shift (2)
AP EAPCET 2021 - 19th August Morning Shift (1)
AP EAPCET Subjects
Physics
Mechanics
Units & Measurement and Dimensions Vector Algebra Motion in a Straight Line Motion in a Plane Circular Motion Laws of Motion Work, Energy and Power Center of Mass and Collision Rotational Motion Elasticity Fluid Mechanics Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Optics
Electromagnetism
Electrostatics Current Electricity Capacitor Moving Charges and Magnetism Magnetism and Matter Electromagnetic Induction Alternating Current Electromagnetic Waves
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Atomic Structure States of Matter Thermodynamics Chemical Equilibrium Ionic Equilibrium Liquid Solution Redox Reactions Surface Chemistry Solid State Electrochemistry Chemical Kinetics
Inorganic Chemistry
Periodic Table and Periodicity Chemical Bonding and Molecular Structure Metallurgy Hydrogen and It's Compounds s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Environmental Chemistry
Organic Chemistry
Mathematics
Algebra
Sets and Relations Logarithms Quadratic Equations Sequences and Series Permutations and Combinations Probability Binomial Theorem Vector Algebra Three Dimensional Geometry Matrices and Determinants Statistics Mathematical Reasoning Complex Numbers
Trigonometry
Trigonometric Ratios & Identities Trigonometric Equations Inverse Trigonometric Functions Properties of Triangles
Calculus
Functions Limits, Continuity and Differentiability Differentiation Application of Derivatives Indefinite Integration Definite Integration Area Under The Curves Differential Equations
Coordinate Geometry



