Thermodynamics · Chemistry · AP EAPCET
MCQ (Single Correct Answer)
Observe the following reaction.
$$ A B \mathrm{O}_3(\mathrm{~s}) \xrightarrow{1000 \mathrm{~K}} A \mathrm{O}(\mathrm{~s})+B \mathrm{O}_2(\mathrm{~g}) $$
$\Delta_r H$ for this reaction is $x \mathrm{~kJ} \mathrm{~mol}^{-1}$. What is its $\Delta_r U$ (in $\mathrm{kJ} \mathrm{mol}^{-1}$ ) at the same temperature?
$$ \left(R=8.3 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}\right) $$
At $300 \mathrm{~K}, \Delta_r G^{\Theta}$ for the reaction $A_2(g) \rightleftharpoons B_2(g)$ is $-11.5 \mathrm{~kJ} \mathrm{~mol}^{-1}$. The Equilibrium constant at 300 K is approximately ( $R=8314 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$ )
Two statements are given below.
Statement I : The reaction $\mathrm{Cr}_2 \mathrm{O}_3+2 \mathrm{Al} \longrightarrow \mathrm{Al}_2 \mathrm{O}_3+2 \mathrm{Cr}$ $\left(\Delta G^{\ominus}=-421 \mathrm{~kJ}\right)$ is thermodynamically feasible.
Statement II : The above reaction occurs at room temperature.
The correct answer is
What is the enthalpy change (in J ) for converting 98 of $\mathrm{H}_2 \mathrm{O}(t)+10^{\circ} \mathrm{C}$ to $\mathrm{H}_2 \mathrm{O}(l)$ at $+20^{\circ} \mathrm{C}$ ?
$$ \left(C_p\left(\mathrm{H}_2 \mathrm{O}(\eta)\right)=75 \mathrm{Jmol}^{-1} \mathrm{~K}^{-1}\right) $$
(density of $\mathrm{H}_2 \mathrm{O}(l)=1 \mathrm{gmL}^{-1}{ }^{})$
$A, B, C$ and $D$ are some compounds. The entnalpy of formation of $A(g), B(g), C(g)$ and $D(g)$ is $9.7,-110,81$ and $-393 \mathrm{~kJ} \mathrm{~mol}^{-1}$ respectively. What is $\Delta_r H$
(in $\mathrm{kJ} \mathrm{mol}^{-1}$ ) for the given reaction ?
$$ A(g)+3 B(g) \longrightarrow C(g)+3 D(g) $$
Observe the following reactions.
$$ \begin{array}{ll} A B(g)+25 \mathrm{H}_2 \mathrm{O}(l) \longrightarrow\left(25 \mathrm{H}_2 \mathrm{O}\right) A B ; & \Delta H=x \mathrm{~kJ} \mathrm{~mol}^{-1} \\ A B(g)+50 \mathrm{H}_2 \mathrm{O}(l) \longrightarrow\left(50 \mathrm{H}_2 \mathrm{O}\right) A B ; & \Delta H=y \mathrm{~kJ} \mathrm{~mol}^{-1} \end{array} $$
Observe the following reaction,
$$ 2 A_2(g)+B_2(g) \xrightarrow{T(\mathrm{~K})} 2 A_2 B(g)+600 \mathrm{~kJ} $$
The standard enthalpy of formation $\left(\Delta_f H^{\ominus}\right)$ of $A_2 B(g)$ is
Given below are two statements :
Statement I For isothermal irreversible change of an ideal gas, $q=-w=p_{\text {ext }}\left(V_{\text {final }}-V_{\text {initial }}\right)$
Statement II For adiabatic change, $\Delta U=w_{\text {adiabatic }}$
The correct answer is :
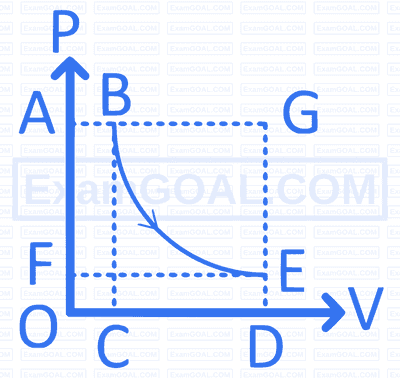
Identify the incorrect statements form the following.
I. $ \Delta S_{\text {pum }}=\left(\Delta S_{\text {nal }}+\Delta S_{\text {um }}\right) $
II. $A(\bar{i} \rightarrow A(\phi)$ : For this process entropy change decreases.
III. Entropy units are $\mathrm{JK} \mathrm{mol}^{-1}$.
Identify the correct statements from the following.
I. At 0 K , the entropy of pure crystalline materials approach zero.
II. Entropy for the process, $$\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{g})$$ decreases.
III. Gibb's energy is a state function.
Use the data from table to estimate the enthalpy of formation of $$\mathrm{CH}_3 \mathrm{CHO}$$.
| Bond enthalpy | Bond | Enthalpy of formation |
|---|---|---|
| $$\mathrm{400~kJ~mol^{-1}}$$ | $$\mathrm{C-H}$$ | $$\mathrm{C}(\mathrm{g}) 700 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ |
| $$\mathrm{350~kJ~mol^{-1}}$$ | $$\mathrm{C-C}$$ | $$\mathrm{H}(\mathrm{g}) 200 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ |
| $$\mathrm{700~kJ~mol^{-1}}$$ | $$\mathrm{C=O}$$ | $$\mathrm{O}(\mathrm{g}) 250 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ |
From the following plots, find the correct option.

Observe the following properties : Volume, enthalpy, density, temperature, heat capacity, pressure and internal energy. The number of extensive properties in the above list is
Match the following.
| A. | Isothermal process | i. | $$ q=\Delta U $$ |
|---|---|---|---|
| B. | Adiabatic process | ii. | $$ W=-p \times \Delta V $$ |
| C. | Isobaric process | iii. | $$ W=\Delta U $$ |
| D. | Isochoric process | iv. | $$ W=-n R T \ln \left(\frac{V_t}{V_i}\right) $$ |
Which of the following expression is correct?
Identify the reaction/process in which the entropy increases.
State $$1 \rightleftharpoons$$ State $$2 \rightleftharpoons$$ State 3 $$\left(\begin{array}{l}T=300 \mathrm{~K} \\ p=15 \mathrm{bar} \\ 1 \mathrm{~mol}\end{array}\right)\left(\begin{array}{l}T=300 \mathrm{~K} \\ p=10 \mathrm{bar} \\ 1 \mathrm{~mol}\end{array}\right)\left(\begin{array}{l}T=300 \mathrm{~K} \\ p=5 \mathrm{bar} \\ 1 \mathrm{~mol}\end{array}\right)$$
Above shows a cyclic process. Calculate the total work done during one complete cycle. [Assume a single step to reach the next state].
For the reaction, $$\mathrm{H}_2 \mathrm{O}(l) \longrightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{g})$$ at $$T=100^{\circ} \mathrm{C}$$ and $$p=1 \mathrm{~atm}$$, choose the correct option.
Two flasks $$A$$ and $$B$$ have equal volumes. $$A$$ is maintained at $$300 \mathrm{~K}$$ and $$B$$ at $$600 \mathrm{~K}$$. Equal masses of $$\mathrm{H}_2$$ and $$\mathrm{CO}_2$$ are taken in flasks $$A$$ and $$B$$ respectively. Find the ratio of total KE of gases in flask $$A$$ to that of $$B$$.
When the temperature of 2 moles of an ideal gas is increased by 20$$^\circ$$C at constant pressure. Find the work involved in the process.
If a chemical reaction is known to be non-spontaneous at 298 K but spontaneous at 350 K, then which among the following conditions is true for the reaction?