Chemical Kinetics and Nuclear Chemistry · Chemistry · JEE Main
MCQ (Single Correct Answer)
In a first order decomposition reaction, the time taken for the decomposition of reactant to one fourth and one eighth of its initial concentration are $t_1$ and $t_2$ (s), respectively. The ratio $t_1/t_2$ will be:
A(g) → B(g) + C(g) is a first order reaction.
| Time | t | ∞ |
|---|---|---|
| Psystem | Pt | P∞ |
The reaction was started with reactant A only. Which of the following expressions is correct for rate constant k?
A person's wound was exposed to some bacteria and then bacterial growth started to happen at the same place. The wound was later treated with some antibacterial medicine and the rate of bacterial decay(r) was found to be proportional with the square of the existing number of bacteria at any instance. Which of the following set of graphs correctly represents the 'before' and 'after' situation of the application of the medicine?
[Given: $N=$ No. of bacteria, $t=$ time, bacterial growth follows $1^{\text {st }}$ order kinetics.]
Reaction $\mathrm{A}(\mathrm{g}) \rightarrow 2 \mathrm{~B}(\mathrm{~g})+\mathrm{C}(\mathrm{g})$ is a first order reaction. It was started with pure A
| t/min | Pressure of system at time t/mm Hg |
|---|---|
| 10 | 160 |
| $\infty$ | 240 |
Which of the following option is incorrect?
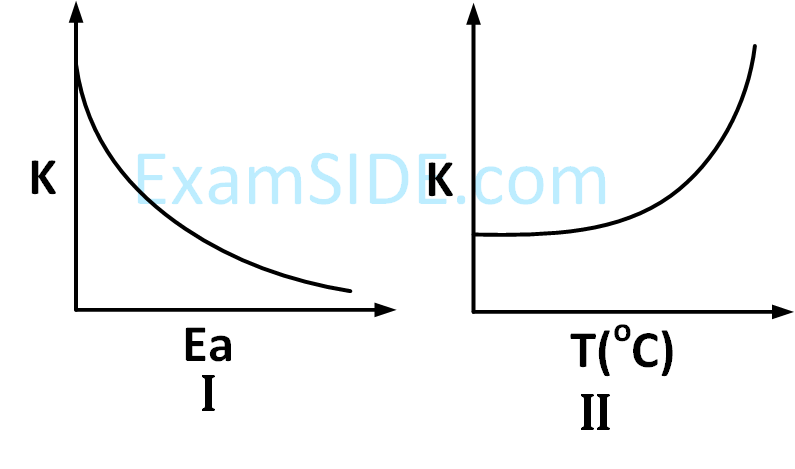
Consider the following plots of $\log$ of rate constant $\mathrm{k}(\log \mathrm{k})$ vs $\frac{1}{\mathrm{~T}}$ for three different reactions. The correct order of activation energies of these reactions is :

Half life of zero order reaction $\mathrm{A} \rightarrow$ product is 1 hour, when initial concentration of reactant is $2.0 \mathrm{~mol} \mathrm{~L}{ }^{-1}$. The time required to decrease concentration of A from 0.50 to $0.25 \mathrm{~mol} \mathrm{~L}^{-1}$ is :
For $\mathrm{A}_2+\mathrm{B}_2 \rightleftharpoons 2 \mathrm{AB}$
$\mathrm{E}_{\mathrm{a}}$ for forward and backward reaction are 180 and $200 \mathrm{~kJ} \mathrm{~mol}^{-1}$ respectively
If catalyst lowers $\mathrm{E}_{\mathrm{a}}$ for both reaction by $100 \mathrm{~kJ} \mathrm{~mol}^{-1}$.
Which of the following statement is correct?
Rate law for a reaction between $A$ and $B$ is given by
$$\mathrm{r}=\mathrm{k}[\mathrm{~A}]^{\mathrm{n}}[\mathrm{~B}]^{\mathrm{m}}$$
If concentration of $A$ is doubled and concentration of $B$ is halved from their initial value, the ratio of new rate of reaction to the initial rate of reaction $\left(\frac{r_2}{r_1}\right)$ is
Consider the following statements related to temperature dependence of rate constants.
Identify the correct statements.
A. The Arrhenius equation holds true only for an elementary homogenous reaction.
B. The unit of $A$ is same as that of $k$ in Arrhenius equation.
C. At a given temperature, a low activation energy means a fast reaction.
D. A and Ea as used in Arrhenius equation depend on temperature.
E. When $\mathrm{Ea} \gg \mathrm{RT}, \mathrm{A}$ and Ea become interdependent.
Choose the correct answer from the options given below:
In a reaction $A+B \rightarrow C$, initial concentrations of $A$ and $B$ are related as $[A]_0=8[B]_0$. The half lives of $A$ and $B$ are 10 min and 40 min , respectively. If they start to disappear at the same time, both following first order kinetics, after how much time will the concentration of both the reactants be same?
Reactant A converts to product D through the given mechanism (with the net evolution of heat):
A → B slow; ΔH = +ve
B → C fast; ΔH = -ve
C → D fast; ΔH = -ve
Which of the following represents the above reaction mechanism?
Drug $X$ becomes ineffective after $50 \%$ decomposition. The original concentration of drug in a bottle was $16 \mathrm{mg} / \mathrm{mL}$ which becomes $4 \mathrm{mg} / \mathrm{mL}$ in 12 months. The expiry time of the drug in months is _________.
Assume that the decomposition of the drug follows first order kinetics.
The reaction $A_2 + B_2 \rightarrow 2AB$ follows the mechanism:
$A_2 \overset{k_1}{\underset{k_{-1}}{\rightleftharpoons}} A + A$ (fast)
$A + B_2 \xrightarrow{k_2} AB + B$ (slow)
$A + B \rightarrow AB$ (fast)
The overall order of the reaction is:
Consider an elementary reaction
$$ \mathrm{A}(\mathrm{~g})+\mathrm{B}(\mathrm{~g}) \rightarrow \mathrm{C}(\mathrm{~g})+\mathrm{D}(\mathrm{~g}) $$
If the volume of reaction mixture is suddenly reduced to $\frac{1}{3}$ of its initial volume, the reaction rate will become ' $x^{\prime}$ times of the original reaction rate. The value of $x$ is :
For bacterial growth in a cell culture, growth law is very similar to the law of radioactive decay. Which of the following graphs is most suitable to represent bacterial colony growth ?
Where N - Number of Bacteria at any time, $\mathrm{N}_0$ - Initial number of Bacteria.

For a given reaction $\mathrm{R} \rightarrow \mathrm{P}, \mathrm{t}_{1 / 2}$ is related to $[\mathrm{A}]_0$ as given in table.
Given: $\log 2=0.30$
Which of the following is true?
A. The order of the reaction is $1 / 2$.
B. If $[\mathrm{A}]_0$ is 1 M , then $\mathrm{t}_{1 / 2}$ is $200 \sqrt{10} \mathrm{~min}$
C. The order of the reaction changes to 1 if the concentration of reactant changes from 0.100 M to 0.500 M.
D. $\mathrm{t}_{1 / 2}$ is 800 min for $[\mathrm{A}]_0=1.6 \mathrm{M}$
Choose the correct answer from the options given below:
Given below are two statements :
Statement (I) :  is valid for first order reaction.
is valid for first order reaction.
Statement (II) :  is valid for first order reaction.
is valid for first order reaction.
In the light of the above statements, choose the correct answer from the options given below :
For a reaction, $\mathrm{N}_2 \mathrm{O}_{5(\mathrm{~g})} \rightarrow 2 \mathrm{NO}_{2(\mathrm{~g})}+\frac{1}{2} \mathrm{O}_{2(\mathrm{~g})}$ in a constant volume container, no products were present initially. The final pressure of the system when $50 \%$ of reaction gets completed is
Which of the following graphs most appropriately represents a zero order reaction?
Consider the given figure and choose the correct option :

Which of the following statement is not true for radioactive decay?
For a reaction $$A \xrightarrow{\mathrm{K}_1} \mathrm{~B} \xrightarrow{\mathrm{K}_2} \mathrm{C}$$ If the rate of formation of B is set to be zero then the concentration of B is given by :
Integrated rate law equation for a first order gas phase reaction is given by (where $$\mathrm{P}_{\mathrm{i}}$$ is initial pressure and $$\mathrm{P}_{\mathrm{t}}$$ is total pressure at time $$t$$)
For a chemical reaction $$\mathrm{A}+\mathrm{B} \rightarrow$$ Product, the order is 1 with respect to $$\mathrm{A}$$ and $$\mathrm{B}$$.
| $$\mathrm{Rate}$$ $$\mathrm{mol~L^{-1}~S^{-1}}$$ |
$$\mathrm{[A]}$$ $$\mathrm{mol~L^{-1}}$$ |
$$\mathrm{[B]}$$ $$\mathrm{mol~L^{-1}}$$ |
|---|---|---|
| 0.10 | 20 | 0.5 |
| 0.40 | $$x$$ | 0.5 |
| 0.80 | 40 | $$y$$ |
What is the value of $$x$$ and $$y$$ ?
The correct reaction profile diagram for a positive catalyst reaction.
Consider the following reaction that goes from A to B in three steps as shown below:

Choose the correct option
A student has studied the decomposition of a gas AB$$_3$$ at 25$$^\circ$$C. He obtained the following data.
| p (mm Hg) | 50 | 100 | 200 | 400 |
|---|---|---|---|---|
| relative t$$_{1/2}$$ (s) | 4 | 2 | 1 | 0.5 |
The order of the reaction is
For kinetic study of the reaction of iodide ion with $$\mathrm{H}_{2} \mathrm{O}_{2}$$ at room temperature :
(A) Always use freshly prepared starch solution.
(B) Always keep the concentration of sodium thiosulphate solution less than that of KI solution.
(C) Record the time immediately after the appearance of blue colour.
(D) Record the time immediately before the appearance of blue colour.
(E) Always keep the concentration of sodium thiosulphate solution more than that of KI solution.
Choose the correct answer from the options given below :
At $$30^{\circ} \mathrm{C}$$, the half life for the decomposition of $$\mathrm{AB}_{2}$$ is $$200 \mathrm{~s}$$ and is independent of the initial concentration of $$\mathrm{AB}_{2}$$. The time required for $$80 \%$$ of the $$\mathrm{AB}_{2}$$ to decompose is
Given: $$\log 2=0.30$$ $$\quad \log 3=0.48$$
For a first order reaction, the time required for completion of 90% reaction is 'x' times the half life of the reaction. The value of 'x' is
(Given : ln 10 = 2.303 and log 2 = 0.3010)
Choose from the options given below, the correct one regarding order of reaction is :
A $$ \to $$ P1 ; B $$ \to $$ P2 ; C $$ \to $$ P3 ; D $$ \to $$ P4,
The order of the above reactions are a, b, c, and d, respectively. The following graph is obtained when log[rate] vs. log[conc.] are plotted

Among the following, the correct sequence for the order of the reactions is :
(R is gas constant)
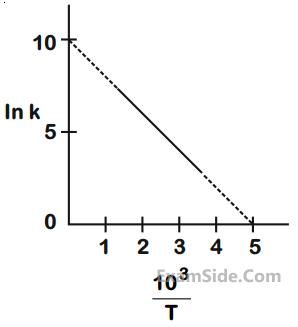
(Use ln 2 = 0.693)
2A + 3B + $${3 \over 2}$$C $$ \to $$ 3P, which statement is correct ?
2A + B $$ \to $$ C + D
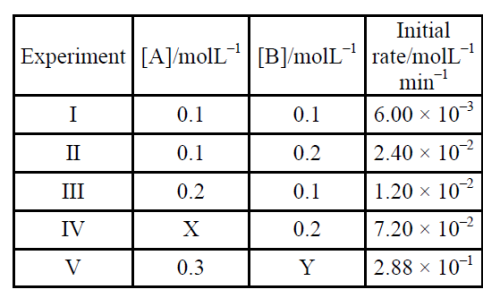
X and Y in the given table are respectively :
$$A\buildrel {700K} \over \longrightarrow {\mathop{\rm Product}\nolimits} $$
$$A\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{catalyst}^{500K}} {\mathop{\rm Product}\nolimits} $$
it was found that Ea is decreased by 30 kJ/mol in the presence of catalyst.
If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre exponential factor is same):

2H2(g) + 2NO(g) $$ \to $$ N2(g) + 2H2O(g)
the observed rate expression is, rate = Kf[NO]2[H2]. The rate expression for the reverse reaction is :
2N2O5(g) $$ \to $$ 4NO2(g) + O2(g).
The initial concentration of N2O5 is 3.00 mol L–1 and it is 2.75 mol L–1 after 30 minutes. The rate of formation of NO2 is :
$${\log _{10}}\left[ { - {{d\left[ A \right]} \over {dt}}} \right] = {\log _{10}}\left[ {{{d\left[ B \right]} \over {dt}}} \right] + 0.3010$$
'A' and 'B' respectively can be :
A+ B $$ \to $$ C + D
Identify the incorrect statement.


if the rate of formation of B is set to be zero then the concentration of B is given by :
| [A] (mol L-1) | [B] (mol L-1) | Initial Rate (mol L-1s-1) |
|---|---|---|
| 0.05 | 0.05 | 0.045 |
| 0.10 | 0.05 | 0.090 |
| 0.20 | 0.10 | 0.72 |
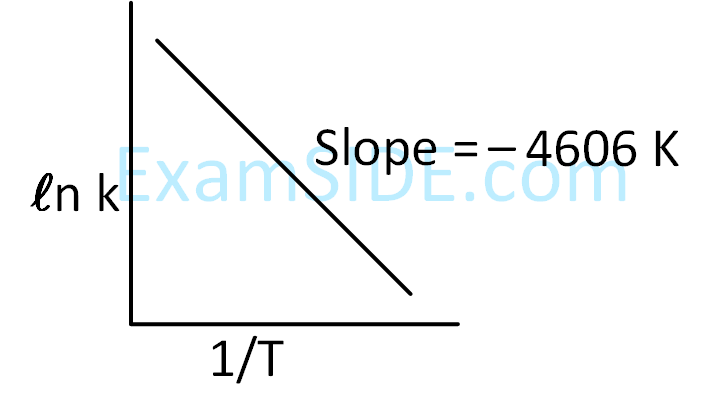
The energy required to active the reactant is :

the expression for $${{d\left[ A \right]} \over {dt}}$$ is
Choose the correct option :
2A + B $$ \to $$ Products
| Experiment | [A] (in mol L$$-$$1) | [b] (in mol L$$-$$1) | Initial Rate of reaction (In mol L$$-$$1 min$$-$$1) |
|---|---|---|---|
| I | 0.10 | 0.20 | 6.93 G 10$$-$$3 |
| II | 0.10 | 0.25 | 6.93 G 10$$-$$3 |
| III | 0.20 | 0.30 | 1.386 G 10$$-$$2 |
The time (in minutes) required to consume half of A is :
(Assume activation energy and preexponential factor are independent of temperature; ln 2 = 0.693; R = 8.314 J mol−1 K−1)
(R = 8.314 J mol–1 K–1)
A + B $$ \to $$ Product
If the concentration of B is increased from 0.1 to 0.3 mole, keeping the value of A at 0.1 mole, the rate constant will be :
O3(g) + Cl$${^ \bullet }$$ (g) $$ \to $$ O2(g) + ClO$${^ \bullet }$$ (g) . . . . . .(i)
ki = 5.2 × 109 L mol−1 s−1
ClO$${^ \bullet }$$(g) + O$${^ \bullet }$$(g) $$ \to $$ O2(g) + Cl$${^ \bullet }$$ (g) . . . . . . (ii)
kii = 2.6 × 1010 L mol−1 s−1
The closest rate constant for the overall reaction O3(g) + O$${^ \bullet }$$ (g) $$ \to $$ 2 O2(g) is :
| Initial Concentration (A) | Initial Concentration (B) | Initial rate of formation of C (mol L-1 s-1) |
|---|---|---|
| 0.1 M | 0.1 M | 1.2 x 10-3 |
| 0.1 M | 0.2 M | 1.2 x 10-3 |
| 0.2 M | 0.1 M | 2.4 x 10-3 |
Consider the reaction :
Cl2(aq) + H2S(aq) → S(s) + 2H+ (aq) + 2Cl– (aq)
The rate equation for this reaction is rate = k [Cl2] [H2S]
Which of these mechanisms is/are consistent with this rate equation?
(A) Cl2 + H2S $$\to$$ H+ + Cl– + Cl+ + HS– (slow)
Cl+ + HS– $$\to$$ H+ + Cl– + S (fast)
(B) H2S $$ \Leftrightarrow $$ H+ + HS– (fast equilibrium)
Cl2 + HS– $$\to$$ 2Cl– + H+ + S (slow)
$$$k = A\,{e^{ - E/RT}}$$$ In this equation, E represents
NO(g) + Br2 (g) $$\leftrightharpoons$$ NOBr2 (g)
NOBr2 (g) + NO (g) $$\to$$ 2NOBr (g)
If the second step is the rate determining step, the order of the reaction with respect to NO(g) is
The reaction must be
$${}_{92}^{238}M \to {}_Y^XN + 2{}_2^4He$$
$${}_Y^XN \to {}_B^AL + 2{\beta ^ + }$$
The number of neutrons in the element L is
2NO(g) + O2(g) $$\to$$ 2NO2(g) volume is suddenly reduce to half its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with respect to NO, the rate of reaction will
Numerical
For the reaction $\mathrm{A} \rightarrow \mathrm{B}$ the following graph was obtained. The time required (in seconds) for the concentration of A to reduce to $2.5 \mathrm{~g} \mathrm{~L}^{-1}$ (if the initial concentration of A was $50 \mathrm{~g} \mathrm{~L}^{-1}$ ) is $\qquad$ . (Nearest integer)
Given : $\log 2=0.3010$

For the reaction A $\to$ products.

The concentration of A at 10 minutes is _________ $\times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1}$ (nearest integer). The reaction was started with $2.5 \mathrm{~mol} \mathrm{~L}^{-1}$ of A .
Consider a complex reaction taking place in three steps with rate constants $\mathrm{k}_1, \mathrm{k}_2$ and $\mathrm{k}_3$ respectively. The overall rate constant $k$ is given by the expression $k=\sqrt{\frac{k_1 k_3}{k_2}}$. If the activation energies of the three steps are 60, 30 and $10 \mathrm{~kJ} \mathrm{~mol}^{-1}$ respectively, then the overall energy of activation in $\mathrm{kJ} \mathrm{mol}^{-1}$ is _________ . (Nearest integer)
For the thermal decomposition of $\mathrm{N}_2 \mathrm{O}_5(\mathrm{~g})$ at constant volume, the following table can be formed, for the reaction mentioned below.
$$2 \mathrm{~N}_2 \mathrm{O}_5(\mathrm{~g}) \rightarrow 2 \mathrm{~N}_2 \mathrm{O}_4(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})$$
| Sr. No. | Time/s | Total pressure/(atm) |
|---|---|---|
| 1 | 0 | 0.6 |
| 2 | 100 | '$\mathrm{x}$' |
$\mathrm{x}=$ __________ $\times 10^{-3} \mathrm{~atm}$ [nearest integer]
Given : Rate constant for the reaction is $4.606 \times 10^{-2} \mathrm{~s}^{-1}$.
$\mathrm{A \rightarrow B}$
The molecule A changes into its isomeric form B by following a first order kinetics at a temperature of 1000 K . If the energy barrier with respect to reactant energy for such isomeric transformation is $191.48 \mathrm{~kJ} \mathrm{~mol}^{-1}$ and the frequency factor is $10^{20}$, the time required for $50 \%$ molecules of A to become B is __________ picoseconds (nearest integer). $\left[\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right]$
Consider the following first order gas phase reaction at constant temperature $$ \mathrm{A}(\mathrm{g}) \rightarrow 2 \mathrm{B}(\mathrm{~g})+\mathrm{C}(\mathrm{g})$$
If the total pressure of the gases is found to be 200 torr after 23 $$\mathrm{sec}$$. and 300 torr upon the complete decomposition of A after a very long time, then the rate constant of the given reaction is ________ $$\times 10^{-2} \mathrm{~s}^{-1}$$ (nearest integer)
[Given : $$\log _{10}(2)=0.301$$]
Given below are two statements :
Statement I : The rate law for the reaction $$A+B \rightarrow C$$ is rate $$(r)=k[A]^2[B]$$. When the concentration of both $$\mathrm{A}$$ and $$\mathrm{B}$$ is doubled, the reaction rate is increased "$$x$$" times.
Statement II : 
The figure is showing "the variation in concentration against time plot" for a "$$y$$" order reaction.
The Value of $$x+y$$ is __________.Consider the following reaction
$$\mathrm{A}+\mathrm{B} \rightarrow \mathrm{C}$$
The time taken for A to become $$1 / 4^{\text {th }}$$ of its initial concentration is twice the time taken to become $$1 / 2$$ of the same. Also, when the change of concentration of B is plotted against time, the resulting graph gives a straight line with a negative slope and a positive intercept on the concentration axis.
The overall order of the reaction is ________.
Consider the two different first order reactions given below
$$\begin{aligned} & \mathrm{A}+\mathrm{B} \rightarrow \mathrm{C} \text { (Reaction 1) } \\ & \mathrm{P} \rightarrow \mathrm{Q} \text { (Reaction 2) } \end{aligned}$$
The ratio of the half life of Reaction 1 : Reaction 2 is $$5: 2$$ If $$t_1$$ and $$t_2$$ represent the time taken to complete $$2 / 3^{\text {rd }}$$ and $$4 / 5^{\text {th }}$$ of Reaction 1 and Reaction 2 , respectively, then the value of the ratio $$t_1: t_2$$ is _________ $$\times 10^{-1}$$ (nearest integer). [Given : $$\log _{10}(3)=0.477$$ and $$\log _{10}(5)=0.699$$]
Time required for $$99.9 \%$$ completion of a first order reaction is _________ times the time required for completion of $$90 \%$$ reaction.(nearest integer)
Consider the following single step reaction in gas phase at constant temperature.
$$2 \mathrm{~A}_{(\mathrm{g})}+\mathrm{B}_{(\mathrm{g})} \rightarrow \mathrm{C}_{(\mathrm{g})}$$
The initial rate of the reaction is recorded as $$\mathrm{r}_1$$ when the reaction starts with $$1.5 \mathrm{~atm}$$ pressure of $$\mathrm{A}$$ and $$0.7 \mathrm{~atm}$$ pressure of B. After some time, the rate $$r_2$$ is recorded when the pressure of C becomes $$0.5 \mathrm{~atm}$$. The ratio $$\mathrm{r}_1: \mathrm{r}_2$$ is _________ $$\times 10^{-1}$$. (Nearest integer)
During Kinetic study of reaction $$\mathrm{2 A+B \rightarrow C+D}$$, the following results were obtained :
| $$\mathrm{A [M]}$$ | $$\mathrm{B [M]}$$ | initial rate of formation of $$\mathrm{D}$$ | |
|---|---|---|---|
| I | 0.1 | 0.1 | $$6.0\times10^{-3}$$ |
| II | 0.3 | 0.2 | $$7.2\times10^{-2}$$ |
| III | 0.3 | 0.4 | $$2.88\times10^{-1}$$ |
| IV | 0.4 | 0.1 | $$2.40\times10^{-2}$$ |
Based on above data, overall order of the reaction is _________.
Consider the following reaction, the rate expression of which is given below
$$\begin{aligned} & \mathrm{A}+\mathrm{B} \rightarrow \mathrm{C} \\ & \text { rate }=\mathrm{k}[\mathrm{A}]^{1 / 2}[\mathrm{~B}]^{1 / 2} \end{aligned}$$
The reaction is initiated by taking $$1 \mathrm{~M}$$ concentration of $$\mathrm{A}$$ and $$\mathrm{B}$$ each. If the rate constant $$(\mathrm{k})$$ is $$4.6 \times 10^{-2} \mathrm{~s}^{-1}$$, then the time taken for $$\mathrm{A}$$ to become $$0.1 \mathrm{~M}$$ is _________ sec. (nearest integer)
Consider the following transformation involving first order elementary reaction in each step at constant temperature as shown below.

Some details of the above reactions are listed below.
| Step | Rate constant (sec$$^{-1}$$) | Activation energy (kJ mol$$^{-1}$$) |
|---|---|---|
| 1 | $$\mathrm{k_1}$$ | 300 |
| 2 | $$\mathrm{k_2}$$ | 200 |
| 3 | $$\mathrm{k_3}$$ | $$\mathrm{Ea_3}$$ |
If the overall rate constant of the above transformation (k) is given as $$\mathrm{k=\frac{k_1 k_2}{k_3}}$$ and the overall activation energy $$(\mathrm{E}_{\mathrm{a}})$$ is $$400 \mathrm{~kJ} \mathrm{~mol} \mathrm{~m}^{-1}$$, then the value of $$\mathrm{Ea}_3$$ is ________ integer)
$\mathrm{A}(\mathrm{g}) \rightarrow 2 \mathrm{~B}(\mathrm{~g})+\mathrm{C}(\mathrm{g})$
| S.No. | Time /s | Total pressure /(atm) |
|---|---|---|
| 1. | 0 | 0.1 |
| 2. | 115 | 0.28 |
The rate constant of the reaction is ________ $\times 10^{-2} \mathrm{~s}^{-1}$ (nearest integer)
$$\mathrm{r}=\mathrm{k}[\mathrm{A}]$$ for a reaction, $$50 \%$$ of $$\mathrm{A}$$ is decomposed in 120 minutes. The time taken for $$90 \%$$ decomposition of $$\mathrm{A}$$ is _________ minutes.
$$\mathrm{NO}_2$$ required for a reaction is produced by decomposition of $$\mathrm{N}_2 \mathrm{O}_5$$ in $$\mathrm{CCl}_4$$ as by equation
$$2 \mathrm{~N}_2 \mathrm{O}_{5(\mathrm{~g})} \rightarrow 4 \mathrm{NO}_{2(\mathrm{~g})}+\mathrm{O}_{2(\mathrm{~g})}$$
The initial concentration of $$\mathrm{N}_2 \mathrm{O}_5$$ is $$3 \mathrm{~mol} \mathrm{~L}^{-1}$$ and it is $$2.75 \mathrm{~mol} \mathrm{~L}^{-1}$$ after 30 minutes.
The rate of formation of $$\mathrm{NO}_2$$ is $$\mathrm{x} \times 10^{-3} \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~min}^{-1}$$, value of $$\mathrm{x}$$ is _________. (nearest integer)
The rate of First order reaction is $$0.04 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$$ at 10 minutes and $$0.03 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{~s}^{-1}$$ at 20 minutes after initiation. Half life of the reaction is _______ minutes.
(Given $$\log 2=0.3010, \log 3=0.4771$$)
The half-life of radioisotope bromine - 82 is 36 hours. The fraction which remains after one day is ________ $$\times 10^{-2}$$.
(Given antilog $$0.2006=1.587$$)
For a reaction taking place in three steps at same temperature, overall rate constant $$\mathrm{K}=\frac{\mathrm{K}_1 \mathrm{~K}_2}{\mathrm{~K}_3}$$. If $$\mathrm{Ea}_1, \mathrm{Ea}_2$$ and $$\mathrm{Ea}_3$$ are 40, 50 and $$60 \mathrm{~kJ} / \mathrm{mol}$$ respectively, the overall $$\mathrm{Ea}$$ is ________ $$\mathrm{kJ} / \mathrm{mol}$$.
Time required for completion of $$99.9 \%$$ of a First order reaction is ________ times of half life $$\left(t_{1 / 2}\right)$$ of the reaction.
Consider the following data for the given reaction
$$2 \mathrm{HI}_{(\mathrm{g})} \rightarrow \mathrm{H}_{2(\mathrm{~g})}+\mathrm{I}_{2(\mathrm{~g})}$$

The order of the reaction is _________.
The activation energy of the catalysed backward reaction is ___________ $\mathrm{kJ}~ \mathrm{mol}^{-1}$.
A(g) $$\to$$ 2B(g) + C(g) is a first order reaction. The initial pressure of the system was found to be 800 mm Hg which increased to 1600 mm Hg after 10 min. The total pressure of the system after 30 min will be _________ mm Hg. (Nearest integer)
$$\mathrm{t}_{87.5}$$ is the time required for the reaction to undergo $$87.5 \%$$ completion and $$\mathrm{t}_{50}$$ is the time required for the reaction to undergo $$50 \%$$ completion. The relation between $$\mathrm{t}_{87.5}$$ and $$\mathrm{t}_{50}$$ for a first order reaction is $$\mathrm{t}_{87.5}=x \times \mathrm{t}_{50}$$ The value of $$x$$ is ___________. (Nearest integer)
The reaction $$2 \mathrm{NO}+\mathrm{Br}_{2} \rightarrow 2 \mathrm{NOBr}$$
takes places through the mechanism given below:
$$\mathrm{NO}+\mathrm{Br}_{2} \Leftrightarrow \mathrm{NOBr}_{2}$$ (fast)
$$\mathrm{NOBr}_{2}+\mathrm{NO} \rightarrow 2 \mathrm{NOBr}$$ (slow)
The overall order of the reaction is ___________.
$$\mathrm{KClO}_{3}+6 \mathrm{FeSO}_{4}+3 \mathrm{H}_{2} \mathrm{SO}_{4} \rightarrow \mathrm{KCl}+3 \mathrm{Fe}_{2}\left(\mathrm{SO}_{4}\right)_{3}+3 \mathrm{H}_{2} \mathrm{O}$$
The above reaction was studied at $$300 \mathrm{~K}$$ by monitoring the concentration of $$\mathrm{FeSO}_{4}$$ in which initial concentration was $$10 \mathrm{M}$$ and after half an hour became 8.8 M. The rate of production of $$\mathrm{Fe}_{2}\left(\mathrm{SO}_{4}\right)_{3}$$ is _________ $$\times 10^{-6} \mathrm{~mol} \mathrm{~L} \mathrm{~s}^{-1}$$ (Nearest integer)
The number of incorrect statement/s from the following is ___________
A. The successive half lives of zero order reactions decreases with time.
B. A substance appearing as reactant in the chemical equation may not affect the rate of reaction
C. Order and molecularity of a chemical reaction can be a fractional number
D. The rate constant units of zero and second order reaction are $$\mathrm{mol} ~\mathrm{L}^{-1} \mathrm{~s}^{-1}$$ and $$\mathrm{mol}^{-1} \mathrm{~L} \mathrm{~s}^{-1}$$ respectively
A molecule undergoes two independent first order reactions whose respective half lives are 12 min and 3 min. If both the reactions are occurring then the time taken for the 50% consumption of the reactant is ___________ min. (Nearest integer)
The number of given statement/s which is/are correct is __________.
(A) The stronger the temperature dependence of the rate constant, the higher is the activation energy.
(B) If a reaction has zero activation energy, its rate is independent of temperature.
(C) The stronger the temperature dependence of the rate constant, the smaller is the activation energy.
(D) If there is no correlation between the temperature and the rate constant then it means that the reaction has negative activation energy.
$$\mathrm{A}$$ $$\rightarrow \mathrm{B}$$
The above reaction is of zero order. Half life of this reaction is $$50 \mathrm{~min}$$. The time taken for the concentration of $$\mathrm{A}$$ to reduce to one-fourth of its initial value is ____________ min. (Nearest integer)
A and B are two substances undergoing radioactive decay in a container. The half life of A is 15 min and that of B is 5 min. If the initial concentration of B is 4 times that of A and they both start decaying at the same time, how much time will it take for the concentration of both of them to be same? _____________ min.
(Given : $\ln 10=2.303$ and $$ \log 2=0.3010 \text { )}$$
A $$\to$$ B
The rate constants of the above reaction at 200 K and 300 K are 0.03 min$$^{-1}$$ and 0.05 min$$^{-1}$$ respectively. The activation energy for the reaction is ___________ J (Nearest integer)
(Given : $$\mathrm{ln10=2.3}$$
$$\mathrm{R=8.3~J~K^{-1}~mol^{-1}}$$
$$\mathrm{\log5=0.70}$$
$$\mathrm{\log3=0.48}$$
$$\mathrm{\log2=0.30}$$)
Given: $\ln 10=2.3 ; \log 2=0.3$
If compound A reacts with B following first order kinetics with rate constant $$2.011 \times 10^{-3} \mathrm{~s}^{-1}$$. The time taken by $$\mathrm{A}$$ (in seconds) to reduce from $$7 \mathrm{~g}$$ to $$2 \mathrm{~g}$$ will be ___________. (Nearest Integer)
$$[\log 5=0.698, \log 7=0.845, \log 2=0.301]$$
For conversion of compound A $$\to$$ B, the rate constant of the reaction was found to be $$\mathrm{4.6\times10^{-5}~L~mol^{-1}~s^{-1}}$$. The order of the reaction is ____________.
For certain chemical reaction $$X\to Y$$, the rate of formation of product is plotted against the time as shown in the figure. The number of $$\mathrm{\underline {correct} }$$ statement/s from the following is ___________.

(A) Over all order of this reaction is one.
(B) Order of this reaction can't be determined.
(C) In region I and III, the reaction is of first and zero order respectively.
(D) In region-II, the reaction is of first order.
(E) In region-II, the order of reaction is in the range of 0.1 to 0.9.
A first order reaction has the rate constant, $$\mathrm{k=4.6\times10^{-3}~s^{-1}}$$. The number of correct statement/s from the following is/are __________
Given : $$\mathrm{\log3=0.48}$$
A. Reaction completes in 1000 s.
B. The reaction has a half-life of 500 s.
C. The time required for 10% completion is 25 times the time required for 90% completion.
D. The degree of dissociation is equal to ($$\mathrm{1-e^{-kt}}$$)
E. The rate and the rate constant have the same unit.
For the first order reaction A $$\to$$ B, the half life is 30 min. The time taken for 75% completion of the reaction is _________ min. (Nearest integer)
Given : log 2 = 0.3010
log 3 = 0.4771
log 5 = 0.6989
The number of correct statement/s from the following is __________
A. Larger the activation energy, smaller is the value of the rate constant.
B. The higher is the activation energy, higher is the value of the temperature coefficient.
C. At lower temperatures, increase in temperature causes more change in the value of k than at higher temperature
D. A plot of $$\mathrm{\ln k}$$ vs $$\frac{1}{T}$$ is a straight line with slope equal to $$-\frac{E_a}{R}$$
Assuming $$1 \,\mu \mathrm{g}$$ of trace radioactive element X with a half life of 30 years is absorbed by a growing tree. The amount of X remaining in the tree after 100 years is ______ $$\times\, 10^{-1} \mu \mathrm{g}$$.
[Given : ln 10 = 2.303; log 2 = 0.30]
The reaction between X and Y is first order with respect to X and zero order with respect to Y.
| Experiment | $${{[X]} \over {mol\,{L^{ - 1}}}}$$ | $${{[Y]} \over {mol\,{L^{ - 1}}}}$$ | $${{Initial\,rate} \over {mol\,{L^{ - 1}}\,{{\min }^{ - 1}}}}$$ |
|---|---|---|---|
| I | 0.1 | 0.1 | $$2 \times {10^{ - 3}}$$ |
| I | L | 0.2 | $$4 \times {10^{ - 3}}$$ |
| III | 0.4 | 0.4 | $$M \times {10^{ - 3}}$$ |
| IV | 0.1 | 0.2 | $$2 \times {10^{ - 3}}$$ |
Examine the data of table and calculate ratio of numerical values of M and L. (Nearest Integer)
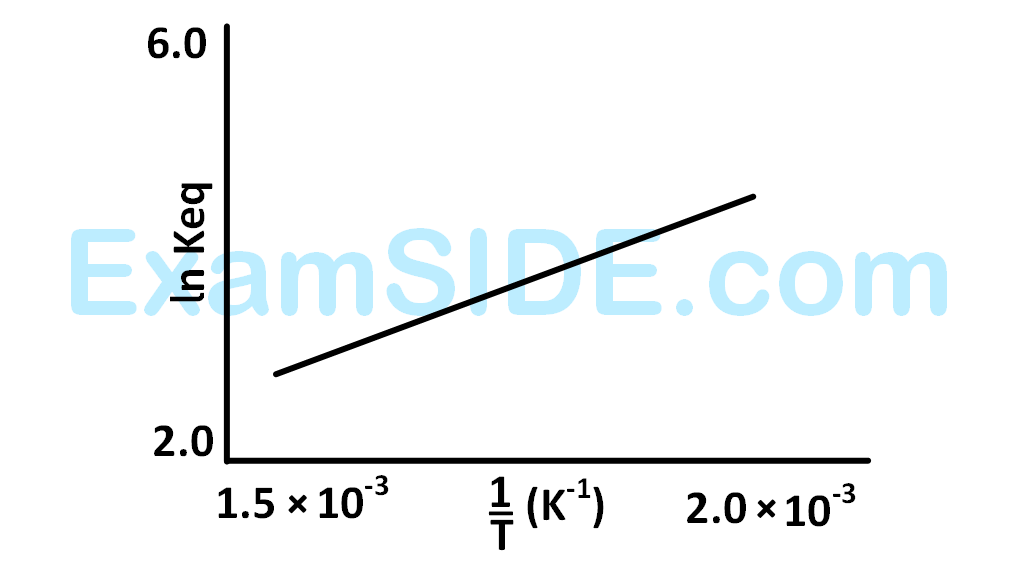
For a reaction, given below is the graph of $$\ln k$$ vs $${1 \over T}$$. The activation energy for the reaction is equal to ____________ $$\mathrm{cal} \,\mathrm{mol}^{-1}$$. (nearest integer)
(Given : $$\mathrm{R}=2 \,\mathrm{cal} \,\mathrm{K}^{-1} \,\mathrm{~mol}^{-1}$$ )

For the given first order reaction
$$\mathrm{A} \rightarrow \mathrm{B}$$
the half life of the reaction is $$0.3010 \mathrm{~min}$$. The ratio of the initial concentration of reactant to the concentration of reactant at time $$2.0 \mathrm{~min}$$ will be equal to ___________. (Nearest integer)
$$\matrix{ {[A]} & \to & {[B]} \cr {{\mathop{\rm Reactant}\nolimits} } & {} & {{\mathop{\rm Product}\nolimits} } \cr } $$
If formation of compound $$[\mathrm{B}]$$ follows the first order of kinetics and after 70 minutes the concentration of $$[\mathrm{A}]$$ was found to be half of its initial concentration. Then the rate constant of the reaction is $$x \times 10^{-6} \mathrm{~s}^{-1}$$. The value of $$x$$ is ______________. (Nearest Integer)
$$2 \mathrm{NO}+2 \mathrm{H}_{2} \rightarrow \mathrm{N}_{2}+2 \mathrm{H}_{2} \mathrm{O}$$
The above reaction has been studied at $$800^{\circ} \mathrm{C}$$. The related data are given in the table below
| Reaction serial number | Initial Pressure of $${H_2}/kPa$$ | Initial Pressure of $$NO/kPa$$ | Initial rate $$\left( {{{ - dp} \over {dt}}} \right)/(kPa/s)$$ |
|---|---|---|---|
| 1 | 65.6 | 40.0 | 0.135 |
| 2 | 65.6 | 20.1 | 0.033 |
| 3 | 38.6 | 65.6 | 0.214 |
| 4 | 19.2 | 65.6 | 0.106 |
The order of the reaction with respect to NO is ___________.
For a reaction $$\mathrm{A} \rightarrow 2 \mathrm{~B}+\mathrm{C}$$ the half lives are $$100 \mathrm{~s}$$ and $$50 \mathrm{~s}$$ when the concentration of reactant $$\mathrm{A}$$ is $$0.5$$ and $$1.0 \mathrm{~mol} \mathrm{~L}^{-1}$$ respectively. The order of the reaction is ______________ . (Nearest Integer)
For the decomposition of azomethane.
CH3N2CH3(g) $$\to$$ CH3CH3(g) + N2(g), a first order reaction, the variation in partial pressure with time at 600 K is given as

The half life of the reaction is __________ $$\times$$ 10$$-$$5 s. [Nearest integer]
The half life for the decomposition of gaseous compound $$\mathrm{A}$$ is $$240 \mathrm{~s}$$ when the gaseous pressure was 500 Torr initially. When the pressure was 250 Torr, the half life was found to be $$4.0$$ min. The order of the reaction is ______________. (Nearest integer)
For the reaction P $$\to$$ B, the values of frequency factor A and activation energy EA are 4 $$\times$$ 1013 s$$-$$1 and 8.3 kJ mol$$-$$1 respectively. If the reaction is of first order, the temperature at which the rate constant is 2 $$\times$$ 10$$-$$6 s$$-$$1 is _____________ $$\times$$ 10$$-$$1 K.
(Given : ln 10 = 2.3, R = 8.3 J K$$-$$1 mol$$-$$1, log2 = 0.30)
The equation
k = (6.5 $$\times$$ 1012s$$-$$1)e$$-$$26000K/T
is followed for the decomposition of compound A. The activation energy for the reaction is ________ kJ mol$$-$$1. [nearest integer]
(Given : R = 8.314 J K$$-$$1 mol$$-$$1)
The activation energy of one of the reactions in a biochemical process is 532611 J mol$$-$$1. When the temperature falls from 310 K to 300 K, the change in rate constant observed is k300 = x $$\times$$ 10$$-$$3 k310. The value of x is ____________.
[Given : $$\ln 10 = 2.3$$, R = 8.3 J K$$-$$1 mol$$-$$1]
A radioactive element has a half life of 200 days. The percentage of original activity remaining after 83 days is ___________. (Nearest integer)
(Given : antilog 0.125 = 1.333, antilog 0.693 = 4.93)
For a first order reaction A $$\to$$ B, the rate constant, k = 5.5 $$\times$$ 10$$-$$14 s$$-$$1. The time required for 67% completion of reaction is x $$\times$$ 10$$-$$1 times the half life of reaction. The value of x is _____________ (Nearest integer)
(Given : log 3 = 0.4771)
It has been found that for a chemical reaction with rise in temperature by 9 K the rate constant gets doubled. Assuming a reaction to be occurring at 300 K, the value of activation energy is found to be ____________ kJ mol$$-$$1. [nearest integer]
(Given ln10 = 2.3, R = 8.3 J K$$-$$1 mol$$-$$1, log 2 = 0.30)
The rate constant for a first order reaction is given by the following equation:
$$\ln k = 33.24 - {{2.0 \times {{10}^4}\,K} \over T}$$
The activation energy for the reaction is given by ____________ kJ mol$$-$$1. (In nearest integer) (Given : R = 8.3 J K$$-$$1 mol$$-$$1)
Catalyst A reduces the activation energy for a reaction by 10 kJ mol$$-$$1 at 300 K. The ratio of rate constants, $${{{}^kT,\,Catalysed} \over {{}^kT,\,Uncatalysed}}$$ is ex. The value of x is ___________. [nearest integer]
[Assume that the pre-exponential factor is same in both the cases. Given R = 8.31 J K$$-$$1 mol$$-$$1]
A flask is filled with equal moles of A and B. The half lives of A and B are 100 s and 50 s respectively and are independent of the initial concentration. The time required for the concentration of A to be four times that of B is ___________ s.
(Given : ln 2 = 0.693)
At 345 K, the half life for the decomposition of a sample of a gaseous compound initially at 55.5 kPa was 340 s. When the pressure was 27.8 kPa, the half life was found to be 170 s. The order of the reaction is ____________. [integer answer]
For a given chemical reaction
$$\gamma$$1A + $$\gamma$$2B $$\to$$ $$\gamma$$3C + $$\gamma$$4D
Concentration of C changes from 10 mmol dm$$-$$3 to 20 mmol dm$$-$$3 in 10 seconds. Rate of appearance of D is 1.5 times the rate of disappearance of B which is twice the rate of disappearance A. The rate of appearance of D has been experimentally determined to be 9 mmol dm$$-$$3 s$$-$$1. Therefore, the rate of reaction is _____________ mmol dm$$-$$3 s$$-$$1. (Nearest Integer)
The rate constants for decomposition of acetaldehyde have been measured over the temperature range 700 - 1000 K. The data has been analysed by plotting ln k vs $${{{{10}^3}} \over T}$$ graph. The value of activation energy for the reaction is ___________ kJ mol$$-$$1. (Nearest integer)
(Given : R = 8.31 J K$$-$$1 mol$$-$$1)

$${\log _{10}}k = 20.35 - {{(2.47 \times {{10}^3})} \over T}$$
The energy of activation in kJ mol$$-$$1 is ____________. (Nearest integer) [Given : R = 8.314 J K$$-$$1 mol$$-$$1]
A + B $$\to$$ M + N in kJ mol$$-$$ is equal to ___________. (Integer answer)

[Given R = 8.31 J K$$-$$ mol$$-$$1; log 6.36 $$\times$$ 10$$-$$3 = $$-$$2.19, 10$$-$$4.79 = 1.62 $$\times$$ 10$$-$$5]
$$2{K_2}C{r_2}{O_7} + 8{H_2}S{O_4} + 3{C_2}{H_6}O \to 2C{r_2}{(S{O_4})_3} + 3{C_2}{H_4}{O_2} + 2{K_2}S{O_4} + 11{H_2}O$$
If the rate of appearance of Cr2(SO4)3 is 2.67 mol min$$-$$1 at a particular time, the rate of disappearance of C2H6O at the same time is _____________ mol min$$-$$1. (Nearest integer)
2NO(g) + 2H2(g) $$\to$$ N2(g) + 2H2O(g)

The order of the reaction with respect to NO is ___________. [Integer answer]
[Use : ln 2 = 0.69, ln 10 = 2.3]
Properties of logarithms : ln xy = y ln x;
$$\ln \left( {{x \over y}} \right) = \ln x - \ln y$$
(Round off to the nearest integer)
In the above first order reaction the initial concentration of N2O5 is 2.40 $$\times$$ 10$$-$$2 mol L$$-$$1 at 318 K. The concentration of N2O5 after 1 hour was 1.60 $$\times$$ 10$$-$$2 mol L$$-$$1. The rate constant of the reaction at 318 K is ______________ $$\times$$ 10$$-$$3 min$$-$$1. (Nearest integer)
[Given : log 3 = 0.477, log 5 = 0.699]
In the above first order reaction the concentration of PCl5 reduces from initial concentration 50 mol L$$-$$1 to 10 mol L$$-$$1 in 120 minutes at 300 K. The rate constant for the reaction at 300 K is x $$\times$$ 10$$-$$2 min$$-$$1. The value of x is __________. [Given log5 = 0.6989]
[Use : ln 10 = 2.303; log10 3 = 0.477; property
of logarithm : log xy = y log x]
This reaction was studied at $$-$$10$$^\circ$$ and the following data was obtained
| Run | $${[NO]_0}$$ | $${[C{l_2}]_0}$$ | $${r_0}$$ |
|---|---|---|---|
| 1 | 0.10 | 0.10 | 0.18 |
| 2 | 0.10 | 0.20 | 0.35 |
| 3 | 0.20 | 0.20 | 1.40 |
$${[NO]_0}$$ and $${[C{l_2}]_0}$$ are the initial concentrations and r0 is the initial reaction rate.
The overall order of the reaction is __________. (Round off to the Nearest Integer).
For a certain quantity of reactants, if the volume of the reaction vessel is reduced by a factor of 3, the rate of the reaction increases by a factor of ____________. (Round off to the Nearest Integer).
products is e$$-$$x. The value of x is __________. (Rounded off to the nearest integer) [Use R = 8.31 J K$$-$$1 mol$$-$$1]
[R = 8.314 J K$$-$$1mol$$-$$1]

The temperature at which the rate constant of the reaction is 10-4 s-1 is _________ K. (Rounded off to the nearest integer)
[Given : The rate constant of the reaction is 10-5 s-1 at 500 K.]
[Assume : ln 10 = 2.303, ln 2 = 0.693]
(Rounded off to the nearest integer)
Take; R = 8.314 J mol–1 K–1 ln 3.555 = 1.268
(Take ln 5 = 1.6094; R = 8.314 J mol–1 K–1)
(Take : log 2 = 0.30; log 2.5 = 0.40)
(Given, R = 8.3 J mol–1 K–1, $$\ln \left( {{3 \over 2}} \right) = 0.4$$, e–3 = 4.0)