1
WB JEE 2022
MCQ (Single Correct Answer)
+1
-0.25

One mole of a diatomic ideal gas undergoes a process shown in P-V diagram. The total heat given to the gas (ln 2 = 0.7) is

2
WB JEE 2022
MCQ (Single Correct Answer)
+2
-0.5

One mole of an ideal monoatomic gas expands along the polytrope PV3 = constant from V1 to V2 at a constant pressure P1. The temperature during the process is such that molar specific heat $${C_V} = {{3R} \over 2}$$. The total heat absorbed during the process can be expressed as
3
WB JEE 2021
MCQ (Single Correct Answer)
+1
-0.25

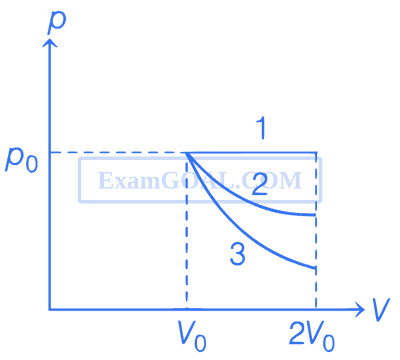
In the given figure, 1 represents isobaric, 2 represents isothermal and 3 represents adiabatic processes of an ideal gas. If $$\Delta$$U1, $$\Delta$$U2 and $$\Delta$$U3 be the changes in internal energy in these processes respectively, then
4
WB JEE 2021
MCQ (Single Correct Answer)
+1
-0.25

If pressure of real gas O2, in a container is given by $$p = {{RT} \over {2V - b}} - {a \over {4{b^2}}}$$, then the mass of the gas in the container is
Questions Asked from Heat and Thermodynamics (MCQ (Single Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
WB JEE Subjects
Physics
Mechanics
Units & Measurements Vector Algebra Motion Laws of Motion Circular Motion Work Power & Energy Center of Mass Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Atomic Structure Redox Reaction Gaseous State Chemical Equilibrium Liquid Solution Ionic Equilibrium Thermodynamics Chemical Kinetics Radioactivity and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table Chemical Bonding Metallurgy Hydrogen and It's Compounds Some s Block Elements Some P Block Elements d and f Block Elements Coordination Compounds
Organic Chemistry
Mathematics
Algebra
Sets and Relations Logarithms Sequence and Series Quadratic Equations Permutations and Combinations Mathematical Induction and Binomial Theorem Mathematical Induction Binomial Theorem Matrices and Determinants Vector Algebra Three Dimensional Geometry Probability Complex Numbers Statistics
Trigonometry
Coordinate Geometry
Calculus