General Organic Chemistry · Chemistry · WB JEE
MCQ (Single Correct Answer)
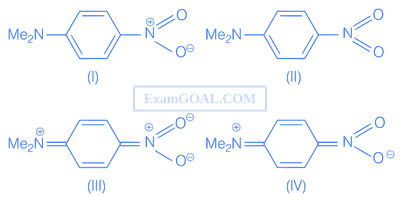
Increasing order of the nucleophilic substitution of following compounds

Arrange the following compounds in order of their increasing acid strength

The correct acidity order of phenol (I), 4-hydroxybenzaldehyde (II) and 3-hydroxybenzaldehyde (III) is

The correct order of C = O bond length in ethyl propanoate (I), ethyl propenoate (II) and ethenyl propanoate (III) is
Select the molecule in which all the atoms may lie on a single plane is
The correct stability order of the following carbocations is
(I) $$\mathrm{{H_2}\mathop C\limits^ \oplus - CH = CH - C{H_3}}$$
(II) $$\mathrm{\mathop C\limits^ \oplus {H_2} - CH = CH - BM{e_2}}$$
(III) $$\mathrm{{H_2}\mathop C\limits^ \oplus - CH = CH - NMe}$$
(IV) $$\mathrm{{H_2}\mathop C\limits^ \oplus - CH = CH - OMe}$$
The correct order of boiling points of N-ethylethanamine (I), ethoxyethane (II) and butan-2-ol (III) is

The correct order of acidity of above compounds is
The correct order of relative stability for the given free radicals is :


Hybridisation of the negative carbons in (1) and (2) are

I. $$^ \oplus C{H_2} - COC{H_3}$$
II. $$^ \oplus C{H_2} - OC{H_3}$$
III. $$^ \oplus C{H_2} - C{H_3}$$

