Heat and Thermodynamics · Physics · WB JEE
MCQ (Single Correct Answer)
Six molecules have speeds 2 units, 5 units, 3 units, 6 units, 3 units and 5 units respectively. The rms speed is
Which one of the figures gives the temperatures dependance of density of water correctly?
An experiment takes 10 minutes to raise temperature of water from 0$$^\circ$$C to 100$$^\circ$$C and another 55 minutes to convert it totally into steam by a stabilized heater. The latent heat of vaporization comes out to be
At what temperature will the rms speed of air molecules be double that of NTP?
When the room temperature becomes equal to the dew point, the relative humidity of the room is
For an ideal gas, a cyclic process ABCA as shown in P-T diagram, when presented in P-V plot, would be

Temperature of a body $\theta$ is slightly more than the temperature of the surrounding $\theta_0$. Its rate of cooling $(R)$ versus temperature of the body $(\theta)$ is plotted. Its shape would be
The speed distribution for a sample of $$\mathrm{N}$$ gas particles is shown below. $$\mathrm{P}(\mathrm{v})=0$$ for $$\mathrm{v}>2 \mathrm{v}_0$$. How many particles have speeds between $$1.2 \mathrm{v}_0$$ and $$1.8 \mathrm{v}_0$$ ?

The internal energy of a thermodynamic system is given by $$U=a s^{4 / 3} V^\alpha$$ where $$\mathrm{s}$$ is entropy, $$\mathrm{V}$$ is volume and '$$\mathrm{a}$$' and '$$\alpha$$' are constants. The value of $$\alpha$$ is
Six molecules of an ideal gas have velocities 1, 3, 5, 5, 6 and 5 m/s respectively. At any given temperature, if $$\mathrm{\overline V}$$ and $$\mathrm{V_{rms}}$$ represent average and rms speed of the molecules, then

A given quantity of gas is taken from A to C in two ways; a) directly from A $$\to$$ C along a straight line and b) in two steps, from A $$\to$$ B and then from B $$\to$$ C. Work done and heat absorbed along the direct path A $$\to$$ C is 200 J and 280 J respectively.
If the work done along A $$\to$$ B $$\to$$ C is 80 J, then heat absorbed along this path is,

Two substances A and B of same mass are heated at constant rate. The variation of temperature $$\theta$$ of the substances with time t is shown in the figure. Choose the correct statement.
Certain amount of an ideal gas is taken from its initial state 1 to final state 4 through the paths 1 $$\to$$ 2 $$\to$$ 3 $$\to$$ 4 as shown in figure. AB, CD, EF are all isotherms. If vp is the most probable speed of the molecules, then

Consider a thermodynamic process where integral energy $$U = A{P^2}V$$ (A = constant). If the process is performed adiabatically, then
One mole of a diatomic ideal gas undergoes a process shown in P-V diagram. The total heat given to the gas (ln 2 = 0.7) is

One mole of an ideal monoatomic gas expands along the polytrope PV3 = constant from V1 to V2 at a constant pressure P1. The temperature during the process is such that molar specific heat $${C_V} = {{3R} \over 2}$$. The total heat absorbed during the process can be expressed as
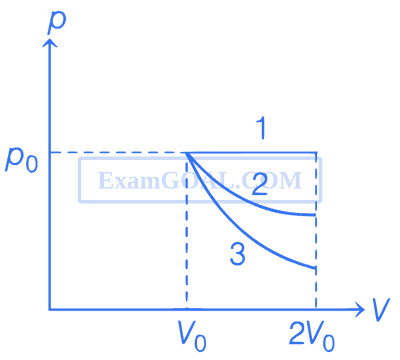
In the given figure, 1 represents isobaric, 2 represents isothermal and 3 represents adiabatic processes of an ideal gas. If $$\Delta$$U1, $$\Delta$$U2 and $$\Delta$$U3 be the changes in internal energy in these processes respectively, then
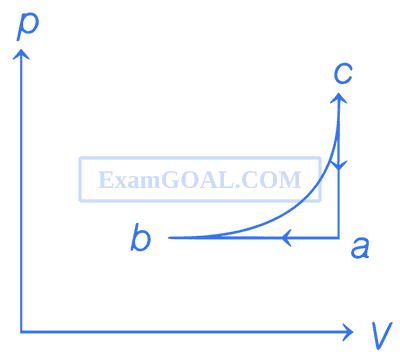
It rejects 50J of heat during ab and absorbs 80J of heat during ca. During bc, there is no transfer of heat and 40J of work is done by the gas. What should be the area of the closed curved abca?

The left compartment is filled with a given mass of an ideal gas of molar mass 32 while the right compartment is filled with an equal mass of another ideal gas of molar mass 18 at same temperature. What will be the distance of P from the left wall A when equilibrium is established?

MCQ (More than One Correct Answer)
Let $\bar{V}, V_{m s}, V_p$ denotes the mean speed, root mean square speed and most probable speed of the molecules each of mass $m$ in an ideal monoatomic gas at absolute temperature $T$ Kelvin. Which statement(s) is/are correct?

A cyclic process is shown in p-v diagram and T-S diagram. Which of the following statements is/are true?
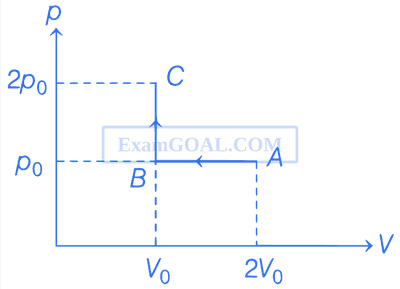
Consider the p - V diagram for 1 mole of an ideal monatomic gas shown in the figure. Which of the following statements is/are true?
"Internal energy of an ideal gas .............."