1
WB JEE 2021
MCQ (More than One Correct Answer)
+2
-0

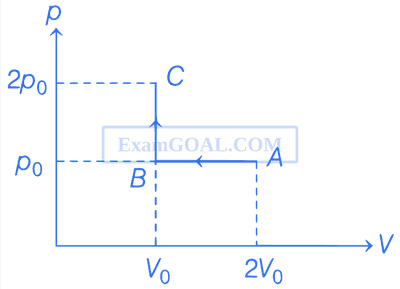
Consider the p - V diagram for 1 mole of an ideal monatomic gas shown in the figure. Which of the following statements is/are true?
2
WB JEE 2019
MCQ (More than One Correct Answer)
+2
-0

The initial pressure and volume of a given mass of an ideal gas with $$\left( {{{{C_p}} \over {{C_V}}} = \gamma } \right)$$, taken in a cylinder fitted with a piston, are p0 and V0 respectively. At this stage the gas has the same temperature as that of the surrounding medium which is T0. It is adiabatically compressed to a volume equal to $${{{V_0}} \over 2}$$. Subsequently the gas is allowed to come to thermal equilibrium with the surroundings. What is the heat released to the surrounding?
3
WB JEE 2018
MCQ (More than One Correct Answer)
+2
-0

Which of the following statement(s) is/are true?
"Internal energy of an ideal gas .............."
"Internal energy of an ideal gas .............."
4
WB JEE 2017
MCQ (More than One Correct Answer)
+2
-0

If the pressure, temperature and density of an ideal gas are denoted by p, T and $$\rho $$, respectively, the velocity of sound in the gas is
Questions Asked from Heat and Thermodynamics (MCQ (Multiple Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
WB JEE Subjects
Physics
Mechanics
Units & Measurements Vector Algebra Motion Laws of Motion Circular Motion Work Power & Energy Center of Mass Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Atomic Structure Redox Reaction Gaseous State Chemical Equilibrium Liquid Solution Ionic Equilibrium Thermodynamics Chemical Kinetics Radioactivity and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table Chemical Bonding Metallurgy Hydrogen and It's Compounds Some s Block Elements Some P Block Elements d and f Block Elements Coordination Compounds
Organic Chemistry
Mathematics
Algebra
Sets and Relations Logarithms Sequence and Series Quadratic Equations Permutations and Combinations Mathematical Induction and Binomial Theorem Mathematical Induction Binomial Theorem Matrices and Determinants Vector Algebra Three Dimensional Geometry Probability Complex Numbers Statistics
Trigonometry
Coordinate Geometry
Calculus