Chemical Bonding & Molecular Structure · Chemistry · JEE Main
MCQ (Single Correct Answer)
In SO2, NO2$-$ and N3$-$, the hybridizations at the central atom are respectively :
Given below are two statements :
Statement (I) :  is more polar than
is more polar than 
Statement (II) : Boiling point of  is lower than
is lower than  but it is more polar than
but it is more polar than  .
.
In the light of the above statements, choose the most appropriate answer from the options given below :
Match the List I with List II.
| List - I Molecule/Ion |
List - II Bond pair : lone pair (on the central atom) |
||
|---|---|---|---|
| (A) | $\mathrm{ICl}_2^{-}$ | (I) | $4:2$ |
| (B) | $\mathrm{H}_2 \mathrm{O}$ | (II) | $4:1$ |
| (C) | $\mathrm{SO_2}$ | (III) | $2:3$ |
| (D) | $\mathrm{XeF_4}$ | (IV) | $2:2$ |
Choose the correct answer from the options given below:
Given below are two statements:
Statement (I): For  , all three possible structures may be drawn as follows.
, all three possible structures may be drawn as follows.

Statement (II): Structure III is most stable, as the orbitals having the lone pairs are axial, where the lp – bp repulsion is minimum.
In the light of the above statements, choose the most appropriate answer from the options given below:
Given below are two statements.

In the light of the above statements, choose the correct answer from the options given below:
Which of the following molecule(s) show/s paramagnetic behavior?
A. $\mathrm{O}_2$
B. $\mathrm{N}_2$
C. $\mathrm{F}_2$
D. $\mathrm{S}_2$
E. $\mathrm{Cl}_2$
Choose the correct answer from the options given below:
Given below are two statements:
Statement I : Wet cotton clothes made of cellulose based carbohydrate takes comparatively longer time to get dried than wet nylon polymer based clothes.
Statement II : Intermolecular hydrogen bonding with water molecule is more in nylon-based clothes than in the case of cotton clothes.
In the light of above statements, choose the correct answer from the options given below
A molecule with the formula $\mathrm{AX}_4 \mathrm{Y}$ has all it's elements from p-block. Element A is rarest, monoatomic, non-radioactive from its group and has the lowest ionization enthalpy value among $\mathrm{A}, \mathrm{X}$ and Y . Elements X and Y have first and second highest electronegativity values respectively among all the known elements. The shape of the molecule is:
Among $\mathrm{SO}_2, \mathrm{NF}_3, \mathrm{NH}_3, \mathrm{XeF}_2, \mathrm{ClF}_3$ and $\mathrm{SF}_4$, the hybridization of the molecule with nonzero dipole moment and highest number of lone-pairs of electrons on the central atom is
Consider the following molecules:

The correct order of rate of hydrolysis is :
Consider ' n ' is the number of lone pair of electrons present in the equatorial position of the most stable structure of $\mathrm{ClF}_3$. The ions from the following with ' n ' number of unpaired electrons are
A. $\mathrm{V}^{3+}$
B. $\mathrm{Ti}^{3+}$
C. $\mathrm{Cu}^{2+}$
D. $\mathrm{Ni}^{2+}$
E. $\mathrm{Ti}^{2+}$
Choose the correct answer from the options given below:
The molecules having square pyramidal geometry are
Given below are two statements :
Statement (I) : Experimentally determined oxygen-oxygen bond lengths in the $\mathrm{O}_3$ are found to be same and the bond length is greater than that of a $\mathrm{O}=\mathrm{O}$ (double bond) but less than that of a single $(\mathrm{O}-\mathrm{O})$ bond.
Statement (II) : The strong lone pair-lone pair repulsion between oxygen atoms is solely responsible for the fact that the bond length in ozone is smaller than that of a double bond $(\mathrm{O}=\mathrm{O})$ but more than that of a single bond $(\mathrm{O}-\mathrm{O})$.
In the light of the above statements, choose the correct answer from the options given below :
Which of the following linear combination of atomic orbitals will lead to formation of molecular orbitals in homonuclear diatomic molecules [internuclear axis in $z$-direction] ?
A. $2 \mathrm{p}_{\mathrm{z}}$ and $2 \mathrm{p}_{\mathrm{x}}$
B. 2 s and $2 \mathrm{p}_{\mathrm{x}}$
C. $3 d_{x y}$ and $3 d_{x^2-y^2}$
D. 2 s and $2 \mathrm{p}_{\mathrm{z}}$
E. $2 p_z$ and $3 d_{x^2-y^2}$
Choose the correct answer from the options given below:
Which of the following statement is true with respect to $\mathrm{H}_2 \mathrm{O}, \mathrm{NH}_3$ and $\mathrm{CH}_4$ ?
A. The central atoms of all the molecules are $\mathrm{sp}^3$ hybridized.
B. The $\mathrm{H}-\mathrm{O}-\mathrm{H}, \mathrm{H}-\mathrm{N}-\mathrm{H}$ and $\mathrm{H}-\mathrm{C}-\mathrm{H}$ angles in the above molecules are $104.5^{\circ}, 107.5^{\circ}$ and $109.5^{\circ}$, respectively.
C. The increasing order of dipole moment is $\mathrm{CH}_4<\mathrm{NH}_3<\mathrm{H}_2 \mathrm{O}$.
D. Both $\mathrm{H}_2 \mathrm{O}$ and $\mathrm{NH}_3$ are Lewis acids and $\mathrm{CH}_4$ is a Lewis base.
E. A solution of $\mathrm{NH}_3$ in $\mathrm{H}_2 \mathrm{O}$ is basic. In this solution $\mathrm{NH}_3$ and $\mathrm{H}_2 \mathrm{O}$ act as Lowry-Bronsted acid and base respectively.
Choose the correct answer from the options given below:
Match the List - I with List - II
| List - I (Classification of molecules based on octet rule) |
List - II (Example) |
||
|---|---|---|---|
| (A) | Molecules obeying octet rule | (I) | $\mathrm{NO}, \mathrm{NO}_2$ |
| (B) | Molecules with incomplete octet | (II) | $\mathrm{BCl}_3, \mathrm{AlCl}_3$ |
| (C) | Molecules with incomplete octet with odd electron | (III) | $\mathrm{H}_2 \mathrm{SO}_4, \mathrm{PCl}_5$ |
| (D) | Molecules with expanded octet | (IV) | $\mathrm{CCl}_4, \mathrm{CO}_2$ |
Choose the correct answer from the options given below:
Arrange the following compounds in increasing order of their dipole moment :
$\mathrm{HBr}, \mathrm{H}_2 \mathrm{~S}, \mathrm{NF}_3$ and $\mathrm{CHCl}_3$
The correct increasing order for bond angles among $$\mathrm{BF}_3, \mathrm{PF}_3$$ and $$\mathrm{ClF}_3$$ is :
In which one of the following pairs the central atoms exhibit $$\mathrm{sp}^2$$ hybridization ?
The shape of carbocation is :
When $$\psi_{\mathrm{A}}$$ and $$\psi_{\mathrm{B}}$$ are the wave functions of atomic orbitals, then $$\sigma^*$$ is represented by :
Match List I with List II
| LIST I (Molecule) |
LIST II (Shape) |
||
|---|---|---|---|
| A. | $$\mathrm{NH_3}$$ | I. | Square pyramid |
| B. | $$\mathrm{BrF_5}$$ | II. | Tetrahedral |
| C. | $$\mathrm{PCl_5}$$ | III. | Trigonal pyramidal |
| D. | $$\mathrm{CH_4}$$ | IV. | Trigonal bipyramidal |
Choose the correct answer from the options given below:
Match List I with List II
| LIST I (Compound/Species) |
LIST II (Shape/Geometry) |
||
|---|---|---|---|
| A. | $$\mathrm{SF_4}$$ | I. | Tetrahedral |
| B. | $$\mathrm{BrF_3}$$ | II. | Pyramidal |
| C. | $$\mathrm{BrO_3^-}$$ | III. | See saw |
| D. | $$\mathrm{NH_4^+}$$ | IV. | Bent T-Shape |
Choose the correct answer from the options given below:
Match List I with List II
| LIST I (Molecule/Species) |
LIST II (Property/Shape) |
||
|---|---|---|---|
| A. | $$\mathrm{SO_2Cl_2}$$ | I. | Paramagnetic |
| B. | $$\mathrm{NO}$$ | II. | Diamagnetic |
| C. | $$\mathrm{NO_2^-}$$ | III. | Tetrahedral |
| D. | $$\mathrm{I_3^-}$$ | IV. | Linear |
Choose the correct answer from the options given below:
Match List I with List II.
| LIST I |
LIST II |
||
|---|---|---|---|
| A. | $$ \mathrm{ICl} $$ |
I. | T - shape |
| B. | $$ \mathrm{ICl}_3 $$ |
II. | Square pyramidal |
| C. | $$ \mathrm{ClF}_5 $$ |
III. | Pentagonal bipyramidal |
| D. | $$ \mathrm{IF}_7 $$ |
IV. | Linear |
Choose the correct answer from the options given below :
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : $$\mathrm{NH}_3$$ and $$\mathrm{NF}_3$$ molecule have pyramidal shape with a lone pair of electrons on nitrogen atom. The resultant dipole moment of $$\mathrm{NH}_3$$ is greater than that of $$\mathrm{NF}_3$$.
Reason (R) : In $$\mathrm{NH}_3$$, the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of the $$\mathrm{N}-\mathrm{H}$$ bonds. $$\mathrm{F}$$ is the most electronegative element.
In the light of the above statements, choose the correct answer from the options given below :
Number of $$\sigma$$ and $$\pi$$ bonds present in ethylene molecule is respectively :
The correct statement/s about Hydrogen bonding is/are
A. Hydrogen bonding exists when H is covalently bonded to the highly electro negative atom.
B. Intermolecular H bonding is present in $$o$$-nitro phenol
C. Intramolecular $$\mathrm{H}$$ bonding is present in HF.
D. The magnitude of $$\mathrm{H}$$ bonding depends on the physical state of the compound.
E. H-bonding has powerful effect on the structure and properties of compounds
Choose the correct answer from the options given below:
The number of species from the following that have pyramidal geometry around the central atom is _________
$$\mathrm{S}_2 \mathrm{O}_3^{2-}, \mathrm{SO}_4^{2-}, \mathrm{SO}_3^{2-}, \mathrm{S}_2 \mathrm{O}_7^{2-}$$
Number of molecules/ions from the following in which the central atom is involved in $$\mathrm{sp}^3$$ hybridization is ________.
$$\mathrm{NO}_3^{-}, \mathrm{BCl}_3, \mathrm{ClO}_2^{-}, \mathrm{ClO}_3^{-}$$
Which one of the following molecules has maximum dipole moment?
Statement (I) : A $\pi$ bonding MO has lower electron density above and below the inter-nuclear axis.
Statement (II) : The $\pi^*$ antibonding MO has a node between the nuclei.
In the light of the above statements, choose the most appropriate answer from the options given below :
Assertion (A): $\mathrm{PH}_3$ has lower boiling point than $\mathrm{NH}_3$.
Reason (R) : In liquid state $\mathrm{NH}_3$ molecules are associated through vander Waal's forces, but $\mathrm{PH}_3$ molecules are associated through hydrogen bonding.
In the light of the above statements, choose the most appropriate answer from the options given below :
Which of the following is least ionic?
The linear combination of atomic orbitals to form molecular orbitals takes place only when the combining atomic orbitals
A. have the same energy
B. have the minimum overlap
C. have same symmetry about the molecular axis
D. have different symmetry about the molecular axis
Choose the most appropriate from the options given below:
Given below are two statements:
Statement - I: Since Fluorine is more electronegative than nitrogen, the net dipole moment of $$\mathrm{NF}_3$$ is greater than $$\mathrm{NH}_3$$.
Statement - II: In $$\mathrm{NH}_3$$, the orbital dipole due to lone pair and the dipole moment of $$\mathrm{NH}$$ bonds are in opposite direction, but in $$\mathrm{NF}_3$$ the orbital dipole due to lone pair and dipole moments of N-F bonds are in same direction.
In the light of the above statements, choose the most appropriate from the options given below:
The molecule / ion with square pyramidal shape is
Aluminium chloride in acidified aqueous solution forms an ion having geometry
Match List I with List II.
| List I Molecule |
List II Shape |
||
|---|---|---|---|
| (A) | $$\mathrm{BrF_5}$$ | (I) | T-Shape |
| (B) | $$\mathrm{H_2O}$$ | (II) | See saw |
| (C) | $$\mathrm{ClF_3}$$ | (III) | Bent |
| (D) | $$\mathrm{SF_4}$$ | (IV) | Square pyramidal |
Choose the correct answer from the options given below :
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : There is a considerable increase in covalent radius from $$\mathrm{N}$$ to $$\mathrm{P}$$. However from As to Bi only a small increase in covalent radius is observed.
Reason (R) : Covalent and ionic radii in a particular oxidation state increases down the group. In the light of the above statements, choose the most appropriate answer from the options given below:
The difference in energy between the actual structure and the lowest energy resonance structure for the given compound is
Choose the polar molecule from the following:
(A) NF3 molecule has a trigonal planar structure.
(B) Bond length of $\mathrm{N}_{2}$ is shorter than $\mathrm{O}_{2}$.
(C) Isoelectronic molecules or ions have identical bond order.
(D) Dipole moment of $\mathrm{H}_{2}\mathrm{S}$ is higher than that of water molecule.
Choose the correct answer from the options given below:
Given below are two statements :
Statement I : $$\mathrm{SO}_{2}$$ and $$\mathrm{H}_{2} \mathrm{O}$$ both possess V-shaped structure.
Statement II : The bond angle of $$\mathrm{SO}_{2}$$ is less than that of $$\mathrm{H}_{2} \mathrm{O}$$.
In the light of the above statements, choose the most appropriate answer from the options given below:
In which of the following processes, the bond order increases and paramagnetic character changes to diamagnetic one ?
The bond order and magnetic property of acetylide ion are same as that of
Match List - I with List - II:
| List - I Species | List - II Geometry/Shape | ||
|---|---|---|---|
| A. | $$\mathrm{H_3O^+}$$ | I. | Tetrahedral |
| B. | Acetylide anion | II. | Linear |
| C. | $$\mathrm{NH_4^+}$$ | III. | Pyramidal |
| D. | $$\mathrm{ClO_2^-}$$ | IV. | Bent |
Choose the correct answer from the options given below:
The compound which does not exist is
Match List I with List II
| LIST I Oxide |
LIST II Type of bond |
||
|---|---|---|---|
| A. | $$\mathrm{N_2O_4}$$ | I. | 1 N = O bond |
| B. | $$\mathrm{NO_2}$$ | II. | 1 N $$-$$ O $$-$$ N bond |
| C. | $$\mathrm{N_2O_5}$$ | III. | 1 N $$-$$ N bond |
| D. | $$\mathrm{N_2O}$$ | IV. | 1 N=N / N $$\equiv$$ N bond |
Choose the correct answer from the options given below:
$$\mathrm{O}-\mathrm{O}$$ bond length in $$\mathrm{H}_{2} \mathrm{O}_{2}$$ is $$\underline{\mathrm{X}}$$ than the $$\mathrm{O}-\mathrm{O}$$ bond length in $$\mathrm{F}_{2} \mathrm{O}_{2}$$. The $$\mathrm{O}-\mathrm{H}$$ bond length in $$\mathrm{H}_{2} \mathrm{O}_{2}$$ is $$\underline{Y}$$ than that of the $$\mathrm{O}-\mathrm{F}$$ bond in $$\mathrm{F}_{2} \mathrm{O}_{2}$$.
Choose the correct option for $$\underline{X}$$ and $$\underline{Y}$$ from those given below :
Match List I with List II
| List I | List II | ||
|---|---|---|---|
| A. | $$\mathrm{XeF_4}$$ | I. | See-saw |
| B. | $$\mathrm{SF_4}$$ | II. | Square-planar |
| C. | $$\mathrm{NH_{4}^{+}}$$ | III. | Bent T-shaped |
| D. | $$\mathrm{BrF_3}$$ | IV. | Tetrahedral |
Choose the correct answer from the options given below :
For $$\mathrm{OF}_{2}$$ molecule consider the following :
A. Number of lone pairs on oxygen is 2 .
B. FOF angle is less than $$104.5^{\circ}$$.
C. Oxidation state of $$\mathrm{O}$$ is $$-2$$.
D. Molecule is bent '$$\mathrm{V}$$' shaped.
E. Molecular geometry is linear.
correct options are:
Match List I with List II
| List I (molecules/ions) |
List II (No. of lone pairs of e$$^-$$ on central atom) |
||
|---|---|---|---|
| A. | $$\mathrm{IF_7}$$ | I. | Three |
| B. | $$\mathrm{ICl}$$$$_4^ - $$ | II. | One |
| C. | $$\mathrm{XeF_6}$$ | III. | Two |
| D. | $$\mathrm{XeF_2}$$ | IV. | Zero |
Choose the correct answer from the options given below :
According to MO theory the bond orders for $$\mathrm{O}$$$$_2^{2 - }$$, $$\mathrm{CO}$$ and $$\mathrm{NO^+}$$ respectively, are
The magnetic behaviour of $$\mathrm{Li_2O,Na_2O_2}$$ and $$\mathrm{KO_2}$$, respectively, are :
The bond dissociation energy is highest for
Statement I : Dipole moment is a vector quantity and by convention it is depicted by a small arrow with tail on the negative centre and head pointing towards the positive centre.
Statement II : The crossed arrow of the dipole moment symbolizes the direction of the shift of charges in the molecules.
In the light of the above statements, choose the most appropriate answer from the options given below :
What is the number of unpaired electron(s) in the highest occupied molecular orbital of the following species : $$\mathrm{{N_2};N_2^ + ;{O_2};O_2^ + }$$ ?
Decreasing order of the hydrogen bonding in following forms of water is correctly represented by
A. Liquid water
B. Ice
C. Impure water
Choose the correct answer from the options given below :
Order of Covalent bond;
$$\mathrm{A.~KF > KI ; LiF > KF}$$
$$\mathrm{B.~KF < KI ; LiF > KF}$$
$$\mathrm{C.~SnCl_4 > SnCl_2 ; CuCl > NaCl}$$
$$\mathrm{D.~LiF > KF ; CuCl < NaCl}$$
$$\mathrm{E.~KF < KI ; CuCl > NaCl}$$
Choose the correct answer from the options given below :
Which of the following pair of molecules contain odd electron molecule and an expanded octet molecule?
Number of lone pairs of electrons in the central atom of $$\mathrm{SCl}_{2}, \mathrm{O}_{3}, \mathrm{ClF}_{3}$$ and $$\mathrm{SF}_{6}$$, respectively, are :
Match List - I with List - II.
| List - I | List - II | ||
|---|---|---|---|
| (A) | $$\psi_{\mathrm{MO}}=\psi_{\mathrm{A}}-\psi_{\mathrm{B}}$$ | (I) | Dipole moment |
| (B) | $$\mu=Q \times r$$ | (II) | Bonding molecular orbital |
| (C) | $$\frac{\mathrm{N}_{\mathrm{b}}-\mathrm{N}_{\mathrm{a}}}{2}$$ | (III) | Anti-bonding molecular orbital |
| (D) | $$\psi_{\mathrm{MO}}=\psi_{\mathrm{A}}+\psi_{\mathrm{B}}$$ | (IV) | Bond order |
Choose the correct answer from the options given below :
Given below are two statements.
Statement I: $$\mathrm{O}_{2}, \mathrm{Cu}^{2+}$$, and $$\mathrm{Fe}^{3+}$$ are weakly attracted by magnetic field and are magnetized in the same direction as magnetic field.
Statement II: $$\mathrm{NaCl}$$ and $$\mathrm{H}_{2} \mathrm{O}$$ are weakly magnetized in opposite direction to magnetic field.
In the light of the above statements, choose the most appropriate answer from the options given below.
Arrange the following in increasing order of their covalent character.
A. $$\mathrm{CaF}_{2}$$
B. $$\mathrm{CaCl}_{2}$$
C. $$\mathrm{CaBr}_{2}$$
D. $$\mathrm{CaI}_{2}$$
Choose the correct answer from the options given below.
Match List-I with List-II :
| List I (Compound) |
List II (Shape) |
||
|---|---|---|---|
| (A) | BrF$$_5$$ | (I) | bent |
| (B) | [CrF$$_6$$]$$^{3 - }$$ | (II) | square pyramidal |
| (C) | O$$_3$$ | (III) | trigonal bipyramidal |
| (D) | PCl$$_5$$ | (IV) | octahedral |
Choose the correct answer from the options given below :
Match List I with List II:
| List I (molecule) |
List II (hybridization ; shape) |
||
|---|---|---|---|
| (A) | XeO$$_3$$ | (I) | sp$$^3$$d ; linear |
| (B) | XeF$$_2$$ | (II) | sp$$^3$$ ; pyramidal |
| (C) | XeOF$$_4$$ | (III) | sp$$^3$$d$$^3$$ ; distorted octahedral |
| (D) | XeF$$_6$$ | (IV) | sp$$^3$$d$$^2$$ ; square pyramidal |
Choose the correct answer from the options given below:
Consider the species CH4, NH$$_4^ + $$ and BH$$_4^ - $$. Choose the correct option with respect to the these species.
Number of lone pair(s) of electrons on central atom and the shape BrF3 molecule respectively, are
In the structure of SF4, the lone pair of electrons on S is in.
Identify the incorrect statement for PCl5 from the following.
The correct order of increasing intermolecular hydrogen bond strength is :
Based upon VSEPR theory, match the shape (geometry) of the molecules in List-I with the molecules in List-II and select the most appropriate option.
| List - I (Shape) |
List - II (Molecules) |
||
|---|---|---|---|
| (A) | T-shaped | (I) | XeF$$_4$$ |
| (B) | Trigonal planar | (II) | SF$$_4$$ |
| (C) | Square planar | (III) | ClF$$_3$$ |
| (D) | See-saw | (IV) | BF$$_3$$ |
Consider the ions/molecule
O$$_2^ + $$, O2, O$$_2^ - $$, O$$_2^ {2-} $$
For increasing bond order the correct option is :
Bonding in which of the following diatomic molecule(s) become(s) stronger, on the basis of MO Theory, by removal of an electron?
(A) NO
(B) N2
(C) O2
(D) C2
(E) B2
Choose the most appropriate answer from the options given below :
Number of electron deficient molecules among the following
PH3, B2H6, CCl4, NH3, LiH and BCl3 is
The correct order of bond orders of $${C_2}^{2 - }$$, $${N_2}^{2 - }$$ and $${O_2}^{2 - }$$
is, respectivelyLi2O, CaO, Na2O2, KO2, MgO and K2O

Choose the most appropriate answer from the options given below :
| List - I (Property) |
List - II (Example) |
||
|---|---|---|---|
| (a) | Diamagnetism | (i) | MnO |
| (b) | Ferrimagnetism | (ii) | $${O_2}$$ |
| (c) | Paramagnetism | (iii) | NaCl |
| (d) | Antiferromagnetism | (iv) | $$F{e_3}{O_4}$$ |
Choose the most appropriate answer from the options given below :
| List-I (Species) |
List-II (Hybrid Orbitals) |
||
|---|---|---|---|
| (a) | $$S{F_4}$$ | (i) | $$s{p^3}{d^2}$$ |
| (b) | $$I{F_5}$$ | (ii) | $${d^2}s{p^3}$$ |
| (c) | $$NO_2^ + $$ | (iii) | $$s{p^3}d$$ |
| (d) | $$NH_4^ + $$ | (iv) | $$s{p^3}$$ |
| (v) | $$sp$$ |
Choose the correct answer from the options given below :
Assertion A : The H$$-$$O$$-$$H bond angle in water molecule is 104.5$$^\circ$$.
Reason R : The lone pair - lone pair repulsion of electrons is higher than the bond pair - bond pair repulsion.
In the light of the above statements, choose the correct answer from the options given below :
| List - I (Molecule) | List - II (Bond order) | ||
|---|---|---|---|
| (a) | $$N{e_2}$$ | (i) | 1 |
| (b) | $${N_2}$$ | (ii) | 2 |
| (c) | $${F_2}$$ | (iii) | 0 |
| (d) | $${O_2}$$ | (iv) | 3 |
Choose the correct answer from the options given below :
Assertion A : Dipole-dipole interactions are the only non-covalent interactions, resulting in hydrogen bond formation.
Reason R : Fluorine is the most electronegative element and hydrogen bonds in HF are symmetrical.
In the light of the above statements, choose the most appropriate answer from the options given below :
A. $$SO_4^{2 - }$$ and $$CrO_4^{2 - }$$
B. SiCl4, and TiCl4
C. NH3 and NO3-
D. BCl3 and BrCl3
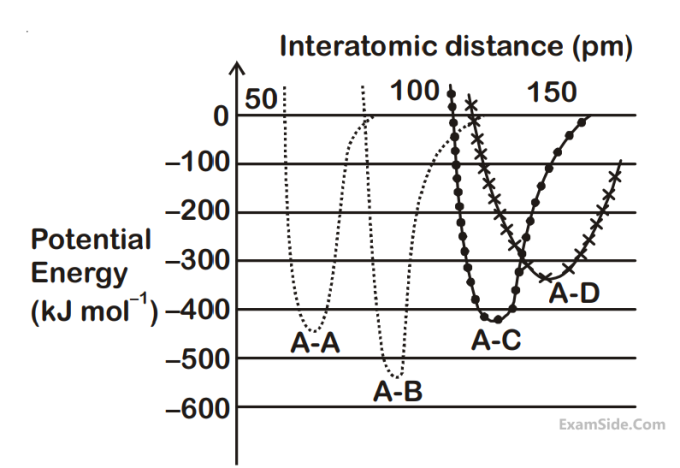
| A | B |
|---|---|
| (i) ion-ion | (a) $${1 \over r}$$ |
| (ii) dipole-dipole | (b) $${1 \over {{r^2}}}$$ |
| (iii) London dispersion | (c) $${1 \over {{r^3}}}$$ |
| (d) $${1 \over {{r^6}}}$$ |
C–Cl, C–Br, C–F, C–I
C2, O2, NO, F2


In hydrogen azide (above) the bond orders of bond (I) and (II) are :
(Atomic nos : B = 5, S = 16, Ni = 28, Xe = 54)
Numerical
Total number of non-bonded electrons present in NO2$-$ ion based on Lewis theory is ______.
The number of molecules/ions that show linear geometry among the following is __________.
$\mathrm{SO}_2, \mathrm{BeCl}_2, \mathrm{CO}_2, \mathrm{~N}_3^{-}, \mathrm{NO}_2, \mathrm{~F}_2 \mathrm{O}, \mathrm{XeF}_2, \mathrm{NO}_2^{+}, \mathrm{I}_3^{-}, \mathrm{O}_3$
Total number of electrons present in $$\left(\pi^*\right)$$ molecular orbitals of $$\mathrm{O}_2, \mathrm{O}_2^{+}$$ and $$\mathrm{O}_2^{-}$$ is ________.
The total number of species from the following in which one unpaired electron is present, is _______.
$$\mathrm{N}_2, \mathrm{O}_2, \mathrm{C}_2^{-}, \mathrm{O}_2^{-}, \mathrm{O}_2^{2-}, \mathrm{H}_2^{+}, \mathrm{CN}^{-}, \mathrm{He}_2^{+}$$
Number of molecules having bond order 2 from the following molecules is _________.
$$\mathrm{C}_2, \mathrm{O}_2, \mathrm{Be}_2, \mathrm{Li}_2, \mathrm{Ne}_2, \mathrm{~N}_2, \mathrm{He}_2$$
Number of molecules from the following which are exceptions to octet rule is _________.
$$\mathrm{CO}_2, \mathrm{NO}_2, \mathrm{H}_2 \mathrm{SO}_4, \mathrm{BF}_3, \mathrm{CH}_4, \mathrm{SiF}_4, \mathrm{ClO}_2, \mathrm{PCl}_5, \mathrm{BeF}_2, \mathrm{C}_2 \mathrm{H}_6, \mathrm{CHCl}_3, \mathrm{CBr}_4$$
Consider the following reactions

The number of protons that do not involve in hydrogen bonding in the product B is _________.
Total number of species from the following with central atom utilising $$\mathrm{sp}^2$$ hybrid orbitals for bonding is ________.
$$\mathrm{NH}_3, \mathrm{SO}_2, \mathrm{SiO}_2, \mathrm{BeCl}_2, \mathrm{C}_2 \mathrm{H}_2, \mathrm{C}_2 \mathrm{H}_4, \mathrm{BCl}_3, \mathrm{HCHO}, \mathrm{C}_6 \mathrm{H}_6, \mathrm{BF}_3, \mathrm{C}_2 \mathrm{H}_4 \mathrm{Cl}_2$$
Number of molecules from the following which can exhibit hydrogen bonding is _________. (nearest integer)

Number of compounds from the following with zero dipole moment is _________.
$$\mathrm{HF}, \mathrm{H}_2, \mathrm{H}_2 \mathrm{~S}, \mathrm{CO}_2, \mathrm{NH}_3, \mathrm{BF}_3, \mathrm{CH}_4, \mathrm{CHCl}_3, \mathrm{SiF}_4, \mathrm{H}_2 \mathrm{O}, \mathrm{BeF}_2$$
In the lewis dot structure for $$\mathrm{NO}_2^{-}$$, total number of valence electrons around nitrogen is _________.
Number of compounds / species from the following with non-zero dipole moment is _________.
$$\mathrm{BeCl}_2, \mathrm{BCl}_3, \mathrm{NF}_3, \mathrm{XeF}_4, \mathrm{CCl}_4, \mathrm{H}_2 \mathrm{O}, \mathrm{H}_2 \mathrm{~S}, \mathrm{HBr}, \mathrm{CO}_2, \mathrm{H}_2, \mathrm{HCl}$$
Number of molecules/species from the following having one unpaired electron is ________.
$$\mathrm{O}_2, \mathrm{O}_2^{-1}, \mathrm{NO}, \mathrm{CN}^{-1}, \mathrm{O}_2^{2-}$$
$\mathrm{PF}_5, \mathrm{BrF}_5, \mathrm{PCl}_5,\left[\mathrm{Pt} \mathrm{Cl}_4\right]^{2-}, \mathrm{BF}_3, \mathrm{Fe}(\mathrm{CO})_5$
A diatomic molecule has a dipole moment of $$1.2 \mathrm{~D}$$. If the bond distance is $$1 \mathrm{~A}^{\circ}$$, then fractional charge on each atom is _________ $$\times 10^{-1}$$ esu.
(Given $$1 \mathrm{~D}=10^{-18}$$ esucm)
The number of species from the following in which the central atom uses $$\mathrm{sp}^3$$ hybrid orbitals in its bonding is __________.
$$\mathrm{NH}_3, \mathrm{SO}_2, \mathrm{SiO}_2, \mathrm{BeCl}_2, \mathrm{CO}_2, \mathrm{H}_2 \mathrm{O}, \mathrm{CH}_4, \mathrm{BF}_3$$
The total number of molecular orbitals formed from $$2 \mathrm{s}$$ and $$2 \mathrm{p}$$ atomic orbitals of a diatomic molecule is __________.
The total number of molecules with zero dipole moment among $$\mathrm{CH}_4, \mathrm{BF}_3, \mathrm{H}_2 \mathrm{O}, \mathrm{HF}, \mathrm{NH}_3, \mathrm{CO}_2$$ and $$\mathrm{SO}_2$$ is ________.
The total number of anti bonding molecular orbitals, formed from $$2 s$$ and $$2 p$$ atomic orbitals in a diatomic molecule is _______.
The number of species from the following which are paramagnetic and with bond order equal to one is _________.
$$\mathrm{H}_2, \mathrm{He}_2^{+}, \mathrm{O}_2^{+}, \mathrm{N}_2^{2-}, \mathrm{O}_2^{2-}, \mathrm{F}_2, \mathrm{Ne}_2^{+}, \mathrm{B}_2$$
Number of compounds with one lone pair of electrons on central atom amongst following is _________.
$$\mathrm{O}_3, \mathrm{H}_2 \mathrm{O}, \mathrm{SF}_4, \mathrm{ClF}_3, \mathrm{NH}_3, \mathrm{BrF}_5, \mathrm{XeF}_4$$
The number of non-polar molecules from the following is _________. $$\mathrm{HF}, \mathrm{H}_2 \mathrm{O}, \mathrm{SO}_2, \mathrm{H}_2, \mathrm{CO}_2, \mathrm{CH}_4, \mathrm{NH}_3, \mathrm{HCl}, \mathrm{CHCl}_3, \mathrm{BF}_3$$
Sum of bond order of CO and NO$$^+$$ is ________.
The maximum number of lone pairs of electrons on the central atom from the following species is ____________.
$$\mathrm{ClO}_{3}{ }^{-}, \mathrm{XeF}_{4}, \mathrm{SF}_{4}$$ and $$\mathrm{I}_{3}{ }^{-}$$
The number of molecules from the following which contain only two lone pair of electrons is ________
$$\mathrm{H}_{2} \mathrm{O}, \mathrm{N}_{2}, \mathrm{CO}, \mathrm{XeF}_{4}, \mathrm{NH}_{3}, \mathrm{NO}, \mathrm{CO}_{2}, \mathrm{~F}_{2}$$
The number of bent-shaped molecule/s from the following is __________
N$$_3^-$$, NO$$_2^-$$, I$$_3^-$$, O$$_3$$, SO$$_2$$
The sum of lone pairs present on the central atom of the interhalogen IF$$_5$$ and IF$$_7$$ is _________
The number of species from the following carrying a single lone pair on central atom Xenon is ___________.
$$\mathrm{XeF}_{5}^{+}, \mathrm{XeO}_{3}, \mathrm{XeO}_{2} \mathrm{~F}_{2}, \mathrm{XeF}_{5}^{-}, \mathrm{XeO}_{3} \mathrm{~F}_{2}, \mathrm{XeOF}_{4}, \mathrm{XeF}_{4}$$
The number of following factors which affect the percent covalent character of the ionic bond is _________
(A) Polarising power of cation
(B) Extent of distortion of anion
(C) Polarisability of the anion
(D) Polarising power of anion
The number of species having a square planar shape from the following is __________.
$$\mathrm{XeF}_{4}, \mathrm{SF}_{4}, \mathrm{SiF}_{4}, \mathrm{BF}_{4}^{-}, \mathrm{BrF}_{4}^{-},\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+},\left[\mathrm{FeCl}_{4}\right]^{2-},\left[\mathrm{PtCl}_{4}\right]^{2-}$$
In an ice crystal, each water molecule is hydrogen bonded to ____________ neighbouring molecules.
The number of species from the following which have square pyramidal structure is _________
$$\mathrm{PF}_{5}, \mathrm{BrF}_{4}^{-}, \mathrm{IF}_{5}, \mathrm{BrF}_{5}, \mathrm{XeOF}_{4}, \mathrm{ICl}_{4}^{-}$$
$\mathrm{XeF}_{2}, \mathrm{I}_{3}^{+}, \mathrm{C}_{3} \mathrm{O}_{2}, \mathrm{I}_{3}^{-}, \mathrm{CO}_{2}, \mathrm{SO}_{2}, \mathrm{BeCl}_{2}$ and $\mathrm{BCl}_{2}^{\ominus}$
The number of molecules or ions from the following, which do not have odd number of electrons are _________.
(A) NO$$_2$$
(B) ICl$$_4^ - $$
(C) BrF$$_3$$
(D) ClO$$_2$$
(E) NO$$_2^ + $$
(F) NO
The total number of lone pairs of electrons on oxygen atoms of ozone is __________.
Consider, $$\mathrm{PF}_{5}, \mathrm{BrF}_{5}, \mathrm{PCl}_{3}, \mathrm{SF}_{6},\left[\mathrm{ICl}_{4}\right]^{-}, \mathrm{ClF}_{3}$$ and $$\mathrm{IF}_{5}$$.
Amongst the above molecule(s)/ion(s), the number of molecule(s)/ion(s) having $$\mathrm{sp}^{3}\mathrm{~d}^{2}$$ hybridisation is __________.
The number of paramagnetic species among the following is ___________.
$$\mathrm{B}_{2}, \mathrm{Li}_{2}, \mathrm{C}_{2}, \mathrm{C}_{2}^{-}, \mathrm{O}_{2}^{2-}, \mathrm{O}_{2}^{+}$$ and $$\mathrm{He}_{2}^{+}$$
The number of interhalogens from the following having square pyramidal structure is :
$$\mathrm{ClF}_{3}, \mathrm{IF}_{7}, \mathrm{BrF}_{5}, \mathrm{BrF}_{3}, \mathrm{I}_{2} \mathrm{Cl}_{6}, \mathrm{IF}_{5}, \mathrm{ClF}, \mathrm{ClF}_{5}$$
The number of molecule(s) or ion(s) from the following having non-planar structure is ____________.
$$\mathrm{NO}_{3}^{-}, \mathrm{H}_{2} \mathrm{O}_{2}, \mathrm{BF}_{3}, \mathrm{PCl}_{3}, \mathrm{XeF}_{4}, \mathrm{SF}_{4}, \mathrm{XeO}_{3}, \mathrm{PH}_{4}^{+}, \mathrm{SO}_{3},\left[\mathrm{Al}(\mathrm{OH})_{4}\right]^{-}$$
Amongst the following, the number of oxide(s) which are paramagnetic in nature is
$$\mathrm{Na}_{2} \mathrm{O}, \mathrm{KO}_{2}, \mathrm{NO}_{2}, \mathrm{~N}_{2} \mathrm{O}, \mathrm{ClO}_{2}, \mathrm{NO}, \mathrm{SO}_{2}, \mathrm{Cl}_{2} \mathrm{O}$$
According to MO theory, number of species/ions from the following having identical bond order is ________.
$$\mathrm{CN}^{-}, \mathrm{NO}^{+}, \mathrm{O}_{2}, \mathrm{O}_{2}^{+}, \mathrm{O}_{2}^{2+}$$
The sum of number of lone pairs of electrons present on the central atoms of XeO3, XeOF4 and XeF6, is ______________
Among the following species
$$\mathrm{N}_{2}, \mathrm{~N}_{2}^{+}, \mathrm{N}_{2}^{-}, \mathrm{N}_{2}^{2-}, \mathrm{O}_{2}, \mathrm{O}_{2}^{+}, \mathrm{O}_{2}^{-}, \mathrm{O}_{2}^{2-}$$
the number of species showing diamagnesim is _______________.
Amongst the following, the number of molecule/(s) having net resultant dipole moment is ____________.
NF3, BF3, BeF2, CHCl3, H2S, SiF4, CCl4, PF5
The hybridization of P exhibited in PF5 is spxdy. The value of y is __________.
Amongst SF4, XeF4, CF4 and H2O, the number of species with two lone pairs of electrons is _____________.
Amongst BeF2, BF3, H2O, NH3, CCl4 and HCl, the number of molecules with non-zero net dipole moment is ____________.
(A) SO3
(B) NO$$_3^ - $$
(C) PCl3
(D) CO$$_3^{2 - }$$
(Round off to the nearest integer)
SF4, BF$$_4^ - $$, ClF3, AsF3, PCl5, BrF5, XeF4, SF6
(A) BF3
(B) SiCl4
(C) PCl5
(D) SF6