1
MHT CET 2025 21st April Evening Shift
MCQ (Single Correct Answer)
+1
-0
Identify correct statement for the reaction.
$$ \mathrm{I}_2+\mathrm{KClO}_3 \xrightarrow{\Delta} \mathrm{ICl}+\mathrm{KIO}_3 $$
2
MHT CET 2025 21st April Evening Shift
MCQ (Single Correct Answer)
+1
-0
Calculate the number of atoms present per unit cell if product of density and volume of unit cell is $1.8 \times 10^{-22} \mathrm{~g}$.
[Mass of an atom $=4.5 \times 10^{-23} \mathrm{~g}$ ]
3
MHT CET 2025 21st April Evening Shift
MCQ (Single Correct Answer)
+1
-0
Calculate the change in internal energy of the system if 25 kJ of work done by the system and it absorbs 10 kJ of heat.
4
MHT CET 2025 21st April Evening Shift
MCQ (Single Correct Answer)
+1
-0
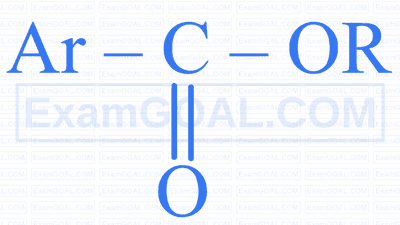
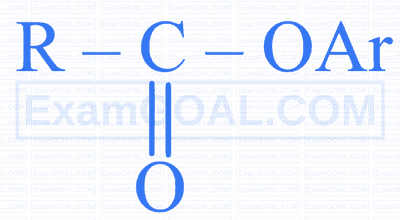
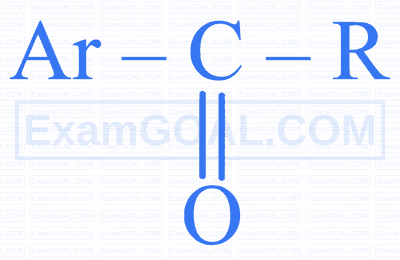
$$ \text { Identify ' } Z \text { ' in the following reaction. } $$

Paper analysis
Total Questions
Chemistry
50
Mathematics
50
Physics
50
More papers of MHT CET
MHT CET 2025 21st April Evening Shift
MHT CET 2025 21st April Morning Shift
MHT CET 2025 20th April Evening Shift
MHT CET 2025 20th April Morning Shift
MHT CET 2025 19th April Evening Shift
MHT CET 2025 19th April Morning Shift
MHT CET 2024 16th May Evening Shift
MHT CET 2024 16th May Morning Shift
MHT CET 2024 15th May Evening Shift
MHT CET 2024 15th May Morning Shift
MHT CET 2024 11th May Evening Shift
MHT CET 2024 11th May Morning Shift
MHT CET 2024 10th May Evening Shift
MHT CET 2024 10th May Morning Shift
MHT CET 2024 9th May Evening Shift
MHT CET 2024 9th May Morning Shift
MHT CET 2024 4th May Evening Shift
MHT CET 2024 4th May Morning Shift
MHT CET 2024 3rd May Evening Shift
MHT CET 2024 3rd May Morning Shift
MHT CET 2024 2nd May Evening Shift
MHT CET 2024 2nd May Morning Shift
MHT CET 2023 14th May Evening Shift
MHT CET 2023 14th May Morning Shift
MHT CET 2023 13th May Evening Shift
MHT CET 2023 13th May Morning Shift
MHT CET 2023 12th May Evening Shift
MHT CET 2023 12th May Morning Shift
MHT CET 2023 11th May Evening Shift
MHT CET 2023 11th May Morning Shift
MHT CET 2023 10th May Evening Shift
MHT CET 2023 10th May Morning Shift
MHT CET 2023 9th May Evening Shift
MHT CET 2023 9th May Morning Shift
MHT CET 2022 11th August Evening Shift
MHT CET 2021 24th September Evening Shift
MHT CET 2021 24th September Morning Shift
MHT CET 2021 23rd September Evening Shift
MHT CET 2021 23th September Morning Shift
MHT CET 2021 22th September Evening Shift
MHT CET 2021 22th September Morning Shift
MHT CET 2021 21th September Evening Shift
MHT CET 2021 21th September Morning Shift
MHT CET 2021 20th September Evening Shift
MHT CET 2021 20th September Morning Shift
MHT CET 2020 19th October Evening Shift
MHT CET 2020 16th October Evening Shift
MHT CET 2020 16th October Morning Shift
MHT CET 2019 3rd May Morning Shift
MHT CET 2019 2nd May Evening Shift
MHT CET 2019 2nd May Morning Shift
MHT CET
Papers
2025
2024
2023
2021
2020