1
AIPMT 2012 Mains
MCQ (Single Correct Answer)
+4
-1

Given that the equilibrium constant for the reaction,
2SO2(g) + O2(g) $$\rightleftharpoons$$ 2SO3(g)
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction at the same temperature ?
SO3(g) $$\rightleftharpoons$$ SO2(g) + $${1 \over 2}$$ O2(g)
2SO2(g) + O2(g) $$\rightleftharpoons$$ 2SO3(g)
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction at the same temperature ?
SO3(g) $$\rightleftharpoons$$ SO2(g) + $${1 \over 2}$$ O2(g)
2
AIPMT 2012 Mains
MCQ (Single Correct Answer)
+4
-1

Four diatomic species are listed below. Identify the correct order in which the bond order is increasing in them
3
AIPMT 2012 Mains
MCQ (Single Correct Answer)
+4
-1

During change of O2 to O$$_2^{ - }$$ ion, the electron adds on which one of the following orbitals ?
4
AIPMT 2012 Mains
MCQ (Single Correct Answer)
+4
-1

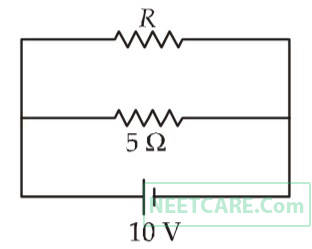
The power dissipated in the circuit shown in the figure is 30 watts. The value of R is

Paper analysis
Total Questions
Biology
56
Chemistry
24
Physics
27
More papers of NEET
NEET 2025
NEET 2024 (Re-Examination)
NEET 2024
NEET 2023 Manipur
NEET 2023
NEET 2022 Phase 2
NEET 2022 Phase 1
NEET 2021
NEET 2020 Phase 1
NEET 2019
NEET 2018
NEET 2017
NEET 2016 Phase 2
NEET 2016 Phase 1
AIPMT 2015
AIPMT 2015 Cancelled Paper
AIPMT 2014
NEET 2013 (Karnataka)
NEET 2013
AIPMT 2012 Mains
AIPMT 2012 Prelims
AIPMT 2011 Mains
AIPMT 2011 Prelims
AIPMT 2010 Mains
AIPMT 2010 Prelims
AIPMT 2009
AIPMT 2008
AIPMT 2007
AIPMT 2006
AIPMT 2005
AIPMT 2004
AIPMT 2003
AIPMT 2002
AIPMT 2001
AIPMT 2000
NEET
Papers
2014
2009
2008
2007
2006
2005
2004
2003
2002
2001
2000