AIPMT 2012 Mains
Paper was held on
Fri, Apr 13, 2012 10:00 AM
Biology
1
Vernalization simulates flowering in
2
Identify the molecules (a) and (b) shown below and select the right option giving their source and use.




3
The idea of mutations was brought forth by :
4
Represented below is the inheritance pattern of the certain type of traits in humans. Which one of the
following conditions could be an example of this pattern ?


5
A test cross is carried out to :
6
The secretory phase in the human menstrual cycle is also called :
7
Plants with ovaries having only one or a few ovules, are generally pollinated by
8
Which one of the following statements is wrong?
9
What is the function of germ pore?
10
Which one of the following characteristics is common both in humans and adult frogs?
11
Which one of the following options gives the correct categorization of six animals according to the type of nitrogenous waste they give out?
12
A fall in glomerular filtration rate (GFR) activates
13
Which one of the following human organs is often called the ''graveyard'' of RBCs?
14
Which one of the following generally acts as an antagonist to gibberellins?
15
Through their effects on plant growth regulators, what do the temperature and light control in the plants?
16
Read the following four statements (A $$-$$ D). phosphorylation involve uphill transport of protons across the membrane.
(A) Both photophosphorylation and oxidative phosphorylation involve uphill transport of protons across the membrane.
(B) In dicot stems, a new cambium originates from cells of pericycle at the time of secondary growth.
(C) Stamens in flowers of Gloriosa and Petunia are polyandrous.
(D) Symbiotic nitrogen fixers occur in free-living state also in soil.
How many of the above statements are right?
(A) Both photophosphorylation and oxidative phosphorylation involve uphill transport of protons across the membrane.
(B) In dicot stems, a new cambium originates from cells of pericycle at the time of secondary growth.
(C) Stamens in flowers of Gloriosa and Petunia are polyandrous.
(D) Symbiotic nitrogen fixers occur in free-living state also in soil.
How many of the above statements are right?
17
Identify the meiotic stage in which the homologous chromosomes separate while the sister chromatids remain associated at their centromeres.
18
Which one of the following biomolecules is correctly characterized?
19
How many plants in the list given below have marginal placentation?
Mustard, Gram, Tulip, Asparagus, Arhar, Sun hemp, Chilli, Colchicum, Onion, Moong, Pea, Tobacco, Lupin
Mustard, Gram, Tulip, Asparagus, Arhar, Sun hemp, Chilli, Colchicum, Onion, Moong, Pea, Tobacco, Lupin
20
Which one of the following organisms is correctly matched with its three characteristic?
21
Which one of the following categories of animals, is correctly described with no single exception in it?
22
Which one of the following pairs of animals are similar to each other pertaining to the feature stated against them?
23
How many organisms in the list given below are autotrophs?
Lactobacillus, Nostoc, Chara, Nitrosomonas, Nitrobacter, Streptomyces, Saccharomyces, Trypanosoma, Porphyra, Wolffia
Lactobacillus, Nostoc, Chara, Nitrosomonas, Nitrobacter, Streptomyces, Saccharomyces, Trypanosoma, Porphyra, Wolffia
24
Read the following five statements (A - E) and answer as asked next to them.
(A) In Equisetum, the female gametophyte is retained on the parent sporophyte.
(B) In Ginkgo, male gametophyte is not independent.
(C) The sporophyte in Riccia is more developed than that in Polytrichum.
(D) Sexual reproduction in Volvox is isogamous.
(E) The spores of slime moulds lack cell walls.
How many of the above statements are correct?
(A) In Equisetum, the female gametophyte is retained on the parent sporophyte.
(B) In Ginkgo, male gametophyte is not independent.
(C) The sporophyte in Riccia is more developed than that in Polytrichum.
(D) Sexual reproduction in Volvox is isogamous.
(E) The spores of slime moulds lack cell walls.
How many of the above statements are correct?
25
Which one of the following pairs is wrongly matched?
26
In the five kingdom classification, Chlamydomonas and Chlorella have been included in
27
The second stage of hydrosere is occupied by plants like
28
Which one of the following is a wrong statement regarding mutations ?
29
Which one of the following statements is
correct with respect to immunity?
30
Read the following four statements (A-D) :
(A) Colostrum is recommended for the new born because it is rich in antigen
(B) Chikengunya is caused by a Gram negative bacterium
(C) Tissue culture has proved useful in obtaining virus-free plants
(D) Beer is manufactured by distillation of fermented grape juice
How many of the above statements are wrong ?
(A) Colostrum is recommended for the new born because it is rich in antigen
(B) Chikengunya is caused by a Gram negative bacterium
(C) Tissue culture has proved useful in obtaining virus-free plants
(D) Beer is manufactured by distillation of fermented grape juice
How many of the above statements are wrong ?
31
Which one of the following pairs of chemical substances, is correctly categarised ?
32
Which one of the following sets of items in the option A – D are correctly categorized with one exception in it ?
33
Identify the human
developmental stage
shown below as well as
the related right place of
its occurrence in a normal pregnant woman,
and select the right option for the two,
together.


34
Which one of the following structures is an organelle within an organelle ?
35
Which one of the following cellular parts is correctly described ?
36
The four sketches (A, B, C and D) given below, represent four different types of animal tissues. Which one
of these is correctly identified in the options given, along with its correct location and function ?


37
The supportive skeletal structures in the human external ears and in the nose tip are examples of
38
Given below is the diagrammatic sketch of a certain type of connective tissue. identify the parts labeled A, B, C and D and select the right option about them


39
As compared to a dicot root, a monocot root has
40
Sacred groves are specially useful in
41
Select the correct statement about biodiversity :
42
Which one of the following organisms is scientifically correctly named, correctly printed according to the international Rules of Nomenclature and correctly described?
43
The rate of formation of new organic matter by rabbit in a grassland, is called :
44
Identify the likely organisms (a), (b), (c) and (d) in the food web shown below :


45
Cuscuta is an example of :
46
Tobacco plants resistant to a nematode have been developed by the introduction of DNA that produced (in the host cells) :
47
The first clinical gene therapy was given for treating -
48
What is it that forms the basis of DNA Fingerprinting ?
49
The figure below shows three steps (A, B, C) of Polymerase Chain Reaction (PCR). Select the option giving correct identification together with what it represents ?


50
In genetic engineering, the antibiotics are used
51
Which one of the following represents a palindromic sequence in DNA ?
52
Biolistics (gene-gun) is suitable for -
53
Consider the following four statements (a-d) and select the option which includes all the correct ones only :
(a) Single cell Spirulina can produce large quantities of food rich in protein, minerals, vitamins etc.
(b) Body weight-wise the microorganisms Methylophilus methylotrophus may be able to produce several times more proteins than the cow per day
(c) Common button mushrooms are a very rich source of vitamin C
(d) A rice variety has been developed which is very rich in calcium
(a) Single cell Spirulina can produce large quantities of food rich in protein, minerals, vitamins etc.
(b) Body weight-wise the microorganisms Methylophilus methylotrophus may be able to produce several times more proteins than the cow per day
(c) Common button mushrooms are a very rich source of vitamin C
(d) A rice variety has been developed which is very rich in calcium
54
The domestic sewage in large cities :
55
Read the following four statements (A-D) :
(A) In transcription, adenosine pairs with uracil
(B) Regulation of lac operon by repressor is referred to as positive regulation
(C) The human genome has approximately 50,000 genes
(D) Haemophilia is a sex-linked recessive disease
How many of the above statements are right ?
(A) In transcription, adenosine pairs with uracil
(B) Regulation of lac operon by repressor is referred to as positive regulation
(C) The human genome has approximately 50,000 genes
(D) Haemophilia is a sex-linked recessive disease
How many of the above statements are right ?
56
In gobar gas, the maximum amount is that of :
Chemistry
1
The orbital angular momentum of a p-electron is given as
2
Red precipitate is obtained when ethanol solution of dimethylglyoxime is added to ammoniacal Ni(II). Which of the following statements is not true?


3
Low spin complex of d6-cation in an octahedral field will have the following energy
4
The catalytic activity of transition metals and their compounds is ascribed mainly to
5
Four successive members of the first series of the transition metals are listed below. For which one of them the standard potential $$\left( {E_{{M^{2 + }}/M}^o} \right)$$ value has a positive sign?
6
Which of the following exhibits only + 3 oxidation state?
7
Which one of the following does not correctly represent the correct order of the property indicated against it?
8
In which of the following arrangements the given sequence is not strictly according to the property indicated against it?
9
Activation energy (E$$a$$) and rate constants (k1 and k2) of a chemical reaction at two different temperatures (T1 and T2) are related by
10
Molar conductivities $$\left( {\Lambda _m^o} \right)$$ at infinite dilution of NaCl, Hcl and CH3COONa are 126.4, 425.9 and 91.0 S cm2 mol$$-$$1 respectively. $$\left( {\Lambda _m^o} \right)$$ for CH3COOH will be
11
The Gibb's energy for the decomposition of Al2O3 at 500oC is as follows
$${2 \over 3}$$ Al2O3 $$ \to $$ $${4 \over 3}$$ Al + O2
$$\Delta $$rG = +960 kJ mol$$-$$1
The potential difference needed for the electrolytic reduction of aluminium oxide (Al2O3) at 500oC is at least
$${2 \over 3}$$ Al2O3 $$ \to $$ $${4 \over 3}$$ Al + O2
$$\Delta $$rG = +960 kJ mol$$-$$1
The potential difference needed for the electrolytic reduction of aluminium oxide (Al2O3) at 500oC is at least
12
Standard reduction potentials of the half reactions are given below :
F2(g) + 2e$$-$$ $$ \to $$ 2F$$-$$(aq) ; Eo = + 2.85 V
Cl2(g) + 2e$$-$$ $$ \to $$ 2Cl$$-$$(aq) ; Eo = + 1.36 V
Br2(l) + 2e$$-$$ $$ \to $$ 2Br$$-$$(aq) ; Eo = + 1.06 V
I2(s) + 2e$$-$$ $$ \to $$ 2I$$-$$(aq) ; Eo = + 0.53 V
The strongest oxidising and reducing agents 23 respectively are
F2(g) + 2e$$-$$ $$ \to $$ 2F$$-$$(aq) ; Eo = + 2.85 V
Cl2(g) + 2e$$-$$ $$ \to $$ 2Cl$$-$$(aq) ; Eo = + 1.36 V
Br2(l) + 2e$$-$$ $$ \to $$ 2Br$$-$$(aq) ; Eo = + 1.06 V
I2(s) + 2e$$-$$ $$ \to $$ 2I$$-$$(aq) ; Eo = + 0.53 V
The strongest oxidising and reducing agents 23 respectively are
13
Vapour pressure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 25oC are 200 mm Hg and 41.5 mm Hg respectively. Vapour pressure of the solution obtained by mixing 25.5 g of CHCl3 and 40 g of CH2Cl2 at the same temperature will be
(Molecular mass of CHCl3 = 119.5 u and molecular mass of CH2Cl2 = 85 u)
(Molecular mass of CHCl3 = 119.5 u and molecular mass of CH2Cl2 = 85 u)
14
An organic compound (C3H9N) (A), when treated with nitrous acid, gave an alcohol and N2 gas was evolved. (A) on warming with CHCl3 and caustic potash gave (C) which on reduction gave isopropylmethylamine. Predict the structure of (A)
15
Consider the following reaction

The product A is

The product A is
16
Which of the following compounds will give a yellow precipitate with iodine and alkali?
17
Consider the reaction :
RCHO + NH2NH2 $$ \to $$ RCH $$=$$ N $$-$$ NH2
What sort of reaction is it?
RCHO + NH2NH2 $$ \to $$ RCH $$=$$ N $$-$$ NH2
What sort of reaction is it?
18
Which of the following compounds can be used as antifreeze in automobile radiators?
19
In the replacement reaction

The reaction will be most favourable if M happens to be

The reaction will be most favourable if M happens to be
20
Which of the following reagents will be able to distinguish between 1-butyne and 2-butyne?
21
Given that the equilibrium constant for the reaction,
2SO2(g) + O2(g) $$\rightleftharpoons$$ 2SO3(g)
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction at the same temperature ?
SO3(g) $$\rightleftharpoons$$ SO2(g) + $${1 \over 2}$$ O2(g)
2SO2(g) + O2(g) $$\rightleftharpoons$$ 2SO3(g)
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction at the same temperature ?
SO3(g) $$\rightleftharpoons$$ SO2(g) + $${1 \over 2}$$ O2(g)
22
Given the reaction between 2 gases represented by A2 and B2 to give the compound AB(g),
A2(g) + B2(g) $$\rightleftharpoons$$ 2AB(g)
At equilibrium, the concentration of
A2 = 3.0 $$ \times $$ 10$$-$$3 M, of B2 = 4.2 $$ \times $$ 10$$-$$3 M, of AB = 2.8 $$ \times $$ 10$$-$$3 M
If the reaction takes place in a sealed vessel at 527oC, then the value of Kc will be
A2(g) + B2(g) $$\rightleftharpoons$$ 2AB(g)
At equilibrium, the concentration of
A2 = 3.0 $$ \times $$ 10$$-$$3 M, of B2 = 4.2 $$ \times $$ 10$$-$$3 M, of AB = 2.8 $$ \times $$ 10$$-$$3 M
If the reaction takes place in a sealed vessel at 527oC, then the value of Kc will be
23
During change of O2 to O$$_2^{ - }$$ ion, the electron adds on which one of the following orbitals ?
24
Four diatomic species are listed below. Identify the correct order in which the bond order is increasing in them
Physics
1
A cell having an emf $$\varepsilon $$ and internal resistance r is connected across a variable external resistance R. As the resistance R is increased, the plot of potential difference V across R is given by
2
To get an output Y= 1 in given circuit which of the following input will be correct ?


3
The transition from the state n = 3 to n = 1 in a hydrogen like atom results in ultraviolet radiation. Infrared radiation will be obtained in the transition from
4
Two radiations of photons energies 1 eV and 2.5 eV, successively illuminate a photosensitive metallic surface of work function 0.5 eV. The ratio of the maximum speeds of the emitted electrons is
5
If the momentum of an electron is changed by P, then the de Broglie wavelength associated with changes by 0.5%. The initial momentum of electron will be
6
A rod of length 10 cm lies along the principal axis of a concave mirror of focal length 10 cm in such a way that its enf closer to the pole is 20 cm away from the mirror. The length of the image is
7
For the angle of minimum deviation of a prism to be equal to its refracting angle, the prism must be made of a material whose refractive index.
8
The ratio of amplitude of magnetic field to the amplitude of electric field for an electromagnetic wave propagatting in vacuum is equal to
9
In a coil of resistance 10 $$\Omega $$, the induced current developed by changing magnetic flux through it, is shown in figure as a function of time. The magnitude of change in flux through the coil in weber is


10
The instantaneous values of alternating current and voltage in a circuit are given as
$$i = {1 \over {\sqrt 2 }}$$ sin (100 $$\pi $$t) ampere
$$e = {1 \over {\sqrt 2 }}\sin \left( {100\pi t + {\pi \over 3}} \right)$$ Volt
The average power in watts consumed in the circuit is
$$i = {1 \over {\sqrt 2 }}$$ sin (100 $$\pi $$t) ampere
$$e = {1 \over {\sqrt 2 }}\sin \left( {100\pi t + {\pi \over 3}} \right)$$ Volt
The average power in watts consumed in the circuit is
11
A magnetic needle suspended parallel to a magnetic field requires $$\sqrt 3 J$$ of work to turn it through 60o. The torque needed to maintain the needle in this position will be
12
A proton carrying 1 MeV kinetic energy is moving in a circular path of radius R in uniform magnetic field. What should be the energy of an $$\alpha $$-particle to describe a circle of same radius in the same field?
13
The power dissipated in the circuit shown in the figure is 30 watts. The value of R is
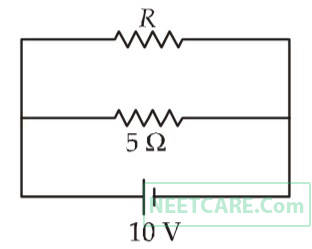

14
Two metallic spheres of radii 1 cm and 3 cm are given charges of $$-$$1 $$ \times $$ 10$$-$$2 C and 5 $$ \times $$ 10$$-$$2 C, respectively. If these are connected by a conducting wire, the final charge on the bigger sphere is
15
A parallel plate capacitor has a uniform electric field E in the space between the plates. If the distance between the plates is d and area of each plate is A, the energy stored in the capacitor is
16
The equation of a simple harmonic wave is given by
y = 3 sin$${\pi \over 2}$$(50t $$-$$ x),
where x and y are in metres and t is in seconds. The ratio of maximum particle velocity to the wave velocity is
y = 3 sin$${\pi \over 2}$$(50t $$-$$ x),
where x and y are in metres and t is in seconds. The ratio of maximum particle velocity to the wave velocity is
17
An ideal gas goes from state A to state B via three different processes as indicated in the P-V diagram.

If Q1, Q2, Q3 indicate the heat absorbed by the gas along the three processes and $$\Delta $$U1, $$\Delta $$U2, $$\Delta $$U3 indicate the change in internal energy along the three processes respectively, then

If Q1, Q2, Q3 indicate the heat absorbed by the gas along the three processes and $$\Delta $$U1, $$\Delta $$U2, $$\Delta $$U3 indicate the change in internal energy along the three processes respectively, then
18
A slab of stone of area 0.36 m2 and thickness 0.1 m is exposed on the lower surface to steam at 100oC. A block of ice at 0oC rests on the upper surface of the slab. In one hour 4.8 kg of ice is melted. The thermal conductivity of slab is
(Given latent heat of fusion of ice = 3.36 $$ \times $$ 105 J kg$$-$$1)
(Given latent heat of fusion of ice = 3.36 $$ \times $$ 105 J kg$$-$$1)
19
Which one of the following plots represents the variation of gravitiational field on a particle with distance r due to a thin spherical shell of radius R? (r is measured from the centre of the spherical shell)
20
If $${v_e}$$ is escape velocity and $${v_o}$$ is orbital velocity of a satellite for orbit close to the earth's surface, then these are related by
21
Three masses are placed on the x-axis : 300 g at origin, 500 g at x = 40 cm and 400 g at x = 70cm. The distance of the centre of mass from the orgin is :
22
The moment of inertia of a uniform circular disc is maximum about an axis perpendicular to the disc and passing through


23
A car of mass m is moving on a level circular track of radius R. If $$\mu $$s represents the static friction between the road and tyres of the car, the maximum speed of the car in circular motion is given by
24
A circular platform is mounted on a frictionless vertical axle. Its radius R = 2 m and its moment of inertia about the axle is 200 kg m2. It is initially at rest. A 50 kg man stands on the edge of the platform and begins to walk along the edge at the speed of 1 ms$$-$$1 relative to the ground. Time taken by the man to complete one revolution is
25
A car of mass m starts from rest and accelerates so that the instantaneous power delivered to the car has a constant magnitude P0. The instantaneous velocity of this car is proportional to
26
A stone is dropped from a height h. It hits the ground with a certain momentum P. If the same stone is dropped from a height 100% more than the previous height, the momentum when it hits the ground will change by
27
The dimensions of $${\left( {{\mu _0}{\varepsilon _0}} \right)^{ - 1/2}}$$ are