AIPMT 2010 Prelims
Paper was held on
Sat, Apr 3, 2010 10:00 AM
Biology
1
Low Ca++ in the body fluid may be the cause of
2
Coiling of garden pea tendrils around any support is an example of
3
What is true about RBCs in humans?
4
Listed below are four respiratory capacities (i $$-$$ iv) and four jumbled respiratory volumes of a normal human adult.
Which one of the following is the correct matching of two capacities and volumes?
| Respiratory capacities |
Respiratory volumes |
||
|---|---|---|---|
| (i) | Residual volume | 2500 mL | |
| (ii) | Vital capacity | 3500 mL | |
| (iii) | Inspiratory reserve volume |
1200 mL | |
| (iv) | Inspiratory capacity | 4500 mL |
Which one of the following is the correct matching of two capacities and volumes?
5
If due to some injury the chordae tendinae of the tricuspid valve of the human heart is partially non-functional, what will be the immediate effect?
6
Which two of the following changes (i $$-$$ iv) usually tend to occur in the plain dwellers when they move to high altitudes (3, 500 m or more)?
(i) Increase in red blood cell size
(ii) Increase in red blood cell production
(iii) Increased breathing rate
(iv) Increase in thrombocyte count
Changes occurring are
(i) Increase in red blood cell size
(ii) Increase in red blood cell production
(iii) Increased breathing rate
(iv) Increase in thrombocyte count
Changes occurring are
7
Which one of the following statements in regard to the excretion by the human kidneys is correct?
8
The principal nitrogenous excretory compound in humans is synthesised
9
Which one of the following is the correct description of a certain part of a normal human skeleton?
10
The nerve centres which control the body temperature and the urge for eating are contained in
11
Injury to adrenal cortex is not likely to affect the secretion of which one of the following?
12
Photoperiodism was first characterised in
13
Which one of the following pairs is incorrectly matched?
14
Toxic agents present in food which interfere with thyroxine synthesis lead to the development of
15
select the correct matching of a hormone, its source and function.
16
Apomictic embryos in Citrus arise form
17
Wind pollinated flowers are
18
Transfer of pollen grains from the another to the stigma of another flower of the same plant is called
19
Sertoli cells are found in :
20
Vasa efferentia are the ductules leading from :
21
The first movements of the foetus and
appearance of hair on its head are usually
observed during which month of pregnancy?
22
Seminal plasma in human males is rich in
23
The second maturation division of the
mammalian ovum occurs
24
Which one of the following statements about
human sperm is correct?
25
Which one of the following statements about certain given animals is correct?
26
Single-celled eukaryotes are included in
27
Virus envelope is known as
28
One of the free-living, anaerobic nitrogen-fixer is
29
Some hyperthermophilic organisms that grow in highly acidic (pH 2) habitats belong to the two groups
30
Membrane-bound organelles are absent in
31
Male and female gametophytes are independent and free-living in
32
Algae have cell wall made up of:
33
One example of animals having a single opening to the outside that serves both as mouth as well as anus is
34
Which one of the following statements about all the four of Spongilla, leech, dolphin and penguin is correct?
35
Which one of the following kinds of animals are triploblastic?
36
Which one of the following statements about
morula in humans is correct?
37
Keel is characteristic of the flowers of
38
Ovary is half-inferior in the flowers of
39
The scutellum observed in a grain of wheat or maize is comparable to which part of the seed in other monocotyledons?
40
The technical term used for the androecium in a flower of China rose (Hibiscus rosa sinensis) is
41
During mitosis, ER and nucleolus begin to disappear at
42
PGA as the first CO2 fixation product was discovered in photosynthesis of
43
C4 plants are more efficient in photosynthesis than C3 plants due to
44
The energy-releasing metabolic process in which substrate is oxidised without an external electron acceptor is called
45
Phototropic curvature is the result of uneven distribution of
46
Which one of the following is an example of ex situ conservation ?
47
Which one of the following is used as vector for
cloning genes into higher organisms ?
48
Restriction endonucleases are enzymes which –
49
Which of the following are used in gene
cloning ?
50
Stirred-tank bioreactors have been designed
for –
51
The genetically-modified (GM) brinjal in India
has been developed for:
52
An improved variety of transgenic basmati rice
53
Genetic engineering has been successfully used
for producing –
54
Some of the characteristics of Bt cotton are –
55
The figure given below is a diagrammatic
representation of response of organisms to
abiotic factors. What do a, b and c represent
respectively –


56
Which one of the following is one of the
characteristics of a biological community?
57
The biomass available for consumption by the
herbivores and the decomposers is called –
58
Study the four statements (a–d) given below and
select the two correct ones out of them –
(a) A lion eating a deer and a sparrow feeding on grain are ecologically similar in being consumers
(b) Predator star fish Pisaster helps in maintaining species diversity of some invertebrates
(c) Predators ultimately lead to the extinction of prey species
(d) Production of chemicals such as nicotine, strychnine by the plants are metaboilic disorders
The two correct stament are-
(a) A lion eating a deer and a sparrow feeding on grain are ecologically similar in being consumers
(b) Predator star fish Pisaster helps in maintaining species diversity of some invertebrates
(c) Predators ultimately lead to the extinction of prey species
(d) Production of chemicals such as nicotine, strychnine by the plants are metaboilic disorders
The two correct stament are-
59
DNA or RNA segment tagged with a radioactive
moleculer is called –
60
Which one of the folliwng is not a lateral
meristem ?
61
Heartwood differs from sapwood in –
62
The chief water conducting elements of xylem
in gymnosperms are :
63
The cells lining the blood vessels belongs to the
category of :
64
The plasma membrane consists mainly of –
65
The main area of variuos types of activites of a
cell is –
66
Which one of the following has its own DNA?
67
Which one of the following structures between
two adjacent cells is an effective transport
pathway?
68
Which stages of cell division do the following
figures A and B represent respectively?


69
Select the two correct statements out of the four
(a-d) statements given below about lac operon.
(a) Glucose or galactose may bind with the repressor and inactivate it
(b) In the absence of lactose the repressor binds with the operator region
(c) The z-gene codes for permease
(d) This was elucidated by Francois Jacob and Jacque Monod
The correct statements are :
(a) Glucose or galactose may bind with the repressor and inactivate it
(b) In the absence of lactose the repressor binds with the operator region
(c) The z-gene codes for permease
(d) This was elucidated by Francois Jacob and Jacque Monod
The correct statements are :
70
The part of Fallopian tube closest to the ovary is
71
In vitro fertilization is a technique that involves
transfer of which one of the following into the
fallopian tube?
72
The permissible use of the technique
aminocentesis is for
73
Cu ions released from copper-releasing Intra
Uterine Devices (IUDs) –
74
Which one of the following symbols and its
representation, used in human pedigree
analysis is correct –
75
ABO blood groups in humans are controlled by
the gene I. It has three alleles – IA, IB
and i.
Since there are three different alleles, six
different genotypes are possible. How many
phenotypes can occur –
76
The genotype of a plant showing the dominant
phenotype can be determined
by –
77
Select the correct statement from the ones given
below with respect to dihybrid cross –
78
Which one of the following cannot be explained
on the basis of Mendel's Law of Dominance?
79
Which one of the following does not follow the
central dogma of molecular biology ?
80
Which one of the following palindromic base
sequences in DNA can be easily cut at about the
middle by some particular restriction enzyme?
81
The one aspect which is not a salient feature of
genetic code, is its being –
82
Darwin's finches are a good example of –
83
Widal test is used for the diagnosis of –
84
Ringworm in humans is caused by :
85
Which one of the following statements is
correct with respect to AIDS ?
86
Infectious proteins are present in –
87
Select the correct statement from the ones given
below :
88
Breeding of crops with high levels of minerals,
vitamins and proteins is called –
89
The common nitrogen-fixer in paddy fields is –
90
Which one of the following not used in organic
farming ?
91
A common biocontrol agent for the control of
plant diseases is –
92
Select the correct statement from the following:
Chemistry
1
Which of the following complex ions is not expected to absorb visible light?
2
Which one of the following complexes is not expected to exhibit isomerism?
3
Crystal field stabilization energy for high spin d4 octahedral complex is
4
An increase in equivalent conductance of a strong electrolyte with dilution is mainly due to
5
Acetamide is treated with the following reagents separately. Which one of these would yield methyl amine?
6
Which of the following reactions will not result in the formation of carbon-carbon bonds?
7
Among the given compounds, the most susceptible to nucleophilic attack at the carbonyl group is
8
Which of the following statements about primary amines is false?
9
Aniline in a set of the following reactions yielded a coloured product Y.

The structure of 'Y' would be

The structure of 'Y' would be
10
Which one of the following does not exhibit the phenomenon of mutaroation?
11
A solution of sucrose (molar mass = 342 g mol$$-$$) has been prepared by dissolving 68.5 g of sucrose in 1000 g of water. The freezing point of the solution obtained will be (Kf for water = 1.86 K kg mol$$-$$1)
12
An aqueous solution is 1.00 molal in KI. Which change will cause the vapour pressure of the solution to increase?
13
For the reduction of silver ions with copper metal, the standard cell potential was found to be + 0.46 V at 25oC. The value of standard Gibb's energy, $$\Delta $$Go will be
(F = 96500 C mol$$-$$1)
(F = 96500 C mol$$-$$1)
14
For the reaction N2O5(g) $$ \to $$ 2NO2(g) + 1/2O2(g)
the value of rate of disappearance of N2O5 is given as 6.25 $$ \times $$ 10$$-$$3 mol L$$-$$1 s$$-$$1. The rate of formation of NO2 and O2 is given respectively as
the value of rate of disappearance of N2O5 is given as 6.25 $$ \times $$ 10$$-$$3 mol L$$-$$1 s$$-$$1. The rate of formation of NO2 and O2 is given respectively as
15
During the kinetic study of the reaction, 2A + B $$ \to $$ C + D, following results were obtained
Based on the above data which one of the following is correct?
| Run | [A]/mol L$$-$$1 | [B]/mol L$$-$$1 | Initial rate of formation of D/mol L$$-$$1 min$$-$$1 |
|---|---|---|---|
| I. | 0.1 | 0.1 | 6.0$$ \times $$10$$-$$3 |
| II. | 0.3 | 0.2 | 7.2$$ \times $$10$$-$$2 |
| III. | 0.3 | 0.4 | 2.88$$ \times $$10$$-$$1 |
| IV. | 0.4 | 0.1 | 2.40$$ \times $$10$$-$$2 |
Based on the above data which one of the following is correct?
16
Which one of the following molecular hydrides acts as a Lewis acid?
17
The tendency of BF3, BCl3 and BBr3 to behave as Lewis acid decreases in the sequence
18
Oxidation states of P in H4P2O5, H4P2O6, H4P2O7 are respectively
19
The correct order of increasing bond angles in the following species is
20
Which of the following ions will exhibit colour in aqueous solutions?
21
Which of the following pairs has the same size?
22
Which of the following ions has electronic configuration [Ar]3d6?
(At. nos. Mn = 25, Fe = 26, Co = 27, Ni = 28)
(At. nos. Mn = 25, Fe = 26, Co = 27, Ni = 28)
23
The existance of two different coloured complexes with the composition of [Co(NH3)4Cl2]+ is due to
24
What is [H+] in mol/L of a solution that is 0.20 M in CH3COONa and 0.10 M in CH3COOH? Ka for CH3COOH = 1.8 $$ \times $$ 10$$-$$5
25
The number of atoms in 0.1 mol of a triatomic gas is (NA = 6.02 $$ \times $$ 1023 mol$$-$$1)
26
25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ion, Na+ and carbonate ions, CO$${}_3^{2 - }$$ are respectively (Molar mass of Na2CO3 = 106 g mol$$-$$1)
27
The correct order of the decreasing ionic radii among the following isoelectronic species is
28
Which of the following represents the correct order of increasing electron gain enthalpy with negative sign for the elements O, S, F and C$$l$$ ?
29
Which one of the following species does not exist under normal conditions?
30
In which of the following pairs of molecules/ ions, the central atoms have sp2 hybridisation ?
31
In which one of the following species the central atom has the type of hybridization which is not the same as that present in the other three ?
32
For an endothermic reaction, energy of activation is Ea and enthalpy of reaction is $$\Delta $$H (both of these in kJ/mol). Minimum value of Ea will be
33
Standard entropies of X2, Y2 and XY3 are 60, 40 and 50 J K$$-$$1 mol$$-$$1 respectively. For the reaction
1/2X2 + 3/2Y2 $$\rightleftharpoons$$ XY3, $$\Delta $$H = $$-$$ 30 kJ,
to be at equilibrium, the temperature should be
1/2X2 + 3/2Y2 $$\rightleftharpoons$$ XY3, $$\Delta $$H = $$-$$ 30 kJ,
to be at equilibrium, the temperature should be
34
If pH of a saturated solution of Ba(OH)2 is 12, the value of its Ksp is
35
In a buffer solution containing equal concentration of B$$-$$ and HB, the Kb for B$$-$$ is 10$$-$$10. The pH of buffer solution is
36
In which of the following equilibrium Kc and Kp are not equal?
37
Which one of the following is most reactive towards electrophilic reagent?
38
In the following the most stable conformation of n-butane is
39
Liquid hydrocarbons can be converted to a mixture of gaseous hydrocarbons by
40
The reaction of toluene with Cl2 in presence of FeCl3 gives X and reaction in presence of light gives Y. Thus, X and Y are
41
In a set of reactions, ethylbenzene yielded a product D.

D would be

D would be
42
Which one is most reactive towards SN1 reaction?
43
The correct order of increasing reactivity of

C$$-$$X bond towards nucleophile in the following compounds is

C$$-$$X bond towards nucleophile in the following compounds is
44
Given are cyclohexanol (I), acetic acid (II), 2, 4, 6-trinitrophenol (III) and phenol (IV). In these the order of decreasing acidic character will be
45
Among the following four compounds
(i) Phenol
(ii) Methyl phenol
(iii) Meta-nitrophenol
(iv) Para-nitrophenol
The acidity order is
(i) Phenol
(ii) Methyl phenol
(iii) Meta-nitrophenol
(iv) Para-nitrophenol
The acidity order is
46
Which of the following compounds has the most acidic nature?
Physics
1
A ray of light travelling in a transparent medium of refractive index $$\mu $$, falls on a surface separating the medium from air at an angle of incidence of 45o. For which of the following value of $$\mu $$ the ray can undergo total internal reflection ?
2
A potentiometer circuit is set up as shown. The potential gradient, across the potentiometer wire, is k volt/cm and the ammeter, present in the circuit, reads 1.0 A when two way key is switched off. The balance points, when the key between the terminals (i) 1 and 2 (ii) 1 and 3, is plugged in, are found to be at length $$l$$1 cm and $$l$$2 cm respectively. The magnitudes, of the resistors R and X, in ohms, are then, equal, respectively, to
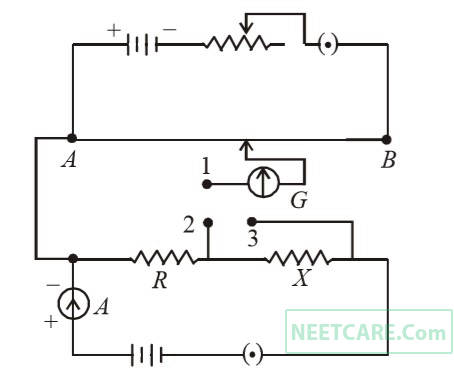

3
Charge q is uniformly spread on a thin ring of radius R. The ring rotates about its axis with a uniform frequency $$f$$ Hz. The magnitude of magnetic induction at the center of the ring is
4
A galvanometer has a coil of resistance 100 ohm and gives a full scale deflection for 30 mA current. If it is to work as a voltmeter of 30 volt range, the resistance required to be added will be
5
A galvanometer has a coil of resistance 100 ohm and gives a full scale deflection for 30 mA current. If it is to work as a voltmeter of 30 volt range, the resistance required to be added will be
6
A square current carrying loop is suspended in a uniform magnetic field acting in the plane of the loop. If the force on one arm of the loop is the net force on the remaining three arms of the loop is
7
Electromagnets are made of soft iron because soft iron has
8
A vibration magnetometer placed in magnetic meridian has a small bar magnet. The magnet executes oscillations with a time period of 2 sec in earth's horizontal magnetic field of 24 microtesla. When a horizontal field of 18 microtesla is produced opposite to the earth's field by placing a current carrying wire, the new time period of magnet will be
9
A conducting circular loop is placed in a uniform magnetic field, B = 0.025 T with its plane perpendicular to the loop. The radius of the loop is made to shrink at a constant rate of 1 mm s$$-$$1. The induced emf when the radius is 2 cm, is
10
In the given circuit the reading of voltmeter V1 and V2 are 300 volts each. The reading of the voltmeter V3 and ammeter A are respectively


11
A 220 volt input is supplied to a transformer. The output circuit draws a current of 2.0 ampere at 440 volts. If the efficiency of the transformer is 80%, the current drwn by the primary windings of the transformer is
12
Which of the following statement is false for the properties of electromagnetic waves ?
13
A lens having focal length f and aperture of diameter d forms an image of intensity I. Aperture of diameter $${d \over 2}$$ in central region of lens is covered by a black paper. Focal length of lens and intensity of image now will be respectively
14
Consider the following two statements.
(A) Kirchoff's junction law follows from the conservation of charge.
(B) Kirchhoff's loop law follows from the conservation of energy.
Which of the following is correct?
(A) Kirchoff's junction law follows from the conservation of charge.
(B) Kirchhoff's loop law follows from the conservation of energy.
Which of the following is correct?
15
A beam of cathode rays is subjected to crossed electric (E) and magnetic fields (B). The fields are adjusted such that the beam is not deflected. The specific charge of the cathode rays is given by
(Where V is the potential difference between cathode and anode)
(Where V is the potential difference between cathode and anode)
16
The potential difference that must be applied to stop the fastest photoelectrons emitted by a nickel surface, having work functions 5.01 eV, when ultraviolet light of 200 nm falls on it, must be
17
A source S1 is producing, 1015 photons per second of wavelength 5000 $$\mathop A\limits^ \circ $$. Another source S2 is producing 1.02 $$ \times $$ 1015 photons per second of wavelength 5100 $$\mathop A\limits^ \circ $$. Then, (power of S2)/(power of S1) is equal to
18
The mass of a $${}_3^7Li$$ Li nucleus is 0.042 u less than the sum of the masses of all its nucleons. The binding energy per nucleon of $${}_3^7Li$$ nucleus is nearly
19
The energy of a hydrogen atom in the ground state is $$-$$ 13.6 eV. The energy of a He+ ion in the first excited state will be
20
The energy of a hydrogen atom in the ground state is $$-$$ 13.6 eV. The energy of a He+ ion in the first excited state will be
21
An alpha nucleus of energy $${1 \over 2}$$ mv2 bombards a heavy nuclear target of charge Ze. Then the distance of closest approach for the alpha nucleus will be proportional to
22
To get an output Y = 1 in given circuit which of the following input will be correct ?


23
Which one of the following statement is false ?
24
The device that can act as a complete electronic circuit is
25
Which one of the following bonds produces a solid that reflects light in the visible region and whose electrical conductivity decreases with temperature and has high melting point ?
26
A man of 50 kg mass is standing in a gravity free space at a height of 10 m above the floor. He throws a stone of 0.5 kg mass downwards with a speed 2 m/s. When the stone reaches the floor, the distance of the man above the floor will be
27
A particle moves a distance x in time t according to equation x = (t + 5)$$-$$1. The acceleration of particle is proportional to
28
A ball is dropped from a high rise platform at t = 0 starting from rest. After 6 seconds another ball is thrown downwards from the same platform with a speed v. The two balls meet at t = 18 s. What is the value of v?
(Take g = 10 m/s2)
(Take g = 10 m/s2)
29
A particle has initial velocity $$\left( {3\widehat i + 4\widehat j} \right)$$ and has acceleration $$\left( {0.4\widehat j + 0.3\widehat j} \right).$$ Its speed after 10 s is
30
Six vectors, $$\overrightarrow a $$ through $$\overrightarrow f $$ have the magnitudes and directions indicated in the figure. Which of the following statements is true ?


31
A block of mass m is in contact with the cart C as shown in the figure.
The coefficient of static friction between the block and the cart is $$\mu $$. The acceleration $$\alpha $$ of the cart that will prevent the block from falling satisfies

The coefficient of static friction between the block and the cart is $$\mu $$. The acceleration $$\alpha $$ of the cart that will prevent the block from falling satisfies

32
A ball moving with velocity 2 m/s collides head on with another stationary ball of double the mass. If the coefficient of restitution is 0.5, then their velocities (in m/s) after collision will be
33
An engine pumps water through a hose pipe. Water passes through the pipe and leaves it with a velocity of 2 m/s. The mass per unit length of water in the pipe is 100 kg/m. What is the power of the engine?
34
A circular disk of moment of inertia $${I_t}$$ is rotating in a horizontal plane, about its symmetry axis, with a constant angular speed $${\omega _i}$$. Another disk of moment of inertia $${I_b}$$ is dropped coaxially onto the rotating disk. Initially the second disk has zero angular speed. Eventually both the disks rotate with a constant angular speed $$\omega $$. The energy lost by the initially rotating disc to friction is
35
Two particles which are initially at rest, move towards each other under the action of their internal attraction. If their speeds are v and 2v at any instant, then the speed of centre of mass of the system will be :
36
A gramophone record is revolving with an angular velocity $$\omega $$. A coin is placed at a distance r from the centre of the record. The static coefficient of friction is $$\mu $$. The coin will revolve with the record if
37
A particle of mass M is situated at the centre of a spherical shell of same mass and radius a. The magnitude of the gravitational potential at a point sutuated at a/2 distance from the centre, will be :
38
The radii of circular orbits of two satellites A and B of the earth, are 4R and R, respectively. If the speed of satellite A is 3V, then the speed of satellite B will be
39
A cylindrical metallic rod in thermal contact with two reservoirs of heat at its two ends conducts an amount of heat Q in time t. The metallic rod is melted and the material is formed into a rod of half the radius of the original rod. What is the amount of heat conducted by the new rod, when placed in thermal contact with the two reservoirs in time t?
40
The total radiant energy per unit area, normal to the direction of incidence, received at a distance R from the centre of a star of radius r, whose outer surface radiates as a black body at a temperature TK is given by
41
Assuming the sun to have a spherical outer surface of radius r, radiating like a black body at temperature toC, the power received by a unit surface, (normal to the incident rays) at a distance R from the centre of the sun is
where $$\sigma $$ is the Stefan's constant.
where $$\sigma $$ is the Stefan's constant.
42
If $$\Delta $$U and $$\Delta $$W represent the increase in internal energy and work done by the system respectively in a thermodynamical process, which of the following is true ?
43
The displacement of a particle along the x-axis is given by x = asin2$$\omega $$t. The motion of the particle corresponds to
44
The period of oscillation of a mass M suspended from a strong of negligible mass is T. If along with it another mass M is also suspended, the period of oscillation will now be
45
A transverse wave is represented by
y = Asin($$\omega $$t $$-$$ kx). For what value of the wavelength is the wave velocity equal to the maximum particle velocity ?
y = Asin($$\omega $$t $$-$$ kx). For what value of the wavelength is the wave velocity equal to the maximum particle velocity ?
46
A tuning fork of frequency 512 Hz makes 4 beats per second with the vibrating string of a piano. The beat frequency decreases to 2 beats per sec when the tension in the piano string is slightly increased. The frequency of the piano string before increasing the tension was
47
A square surface of side L meter in the plane of the paper is placed in a uniform electric field $$E$$(volt/m) acting along the same plane at an angle $$\theta $$ with the horizontal side of the square as shown in figurre.

The electric flux linked to the surface, in units of volt m is

The electric flux linked to the surface, in units of volt m is
48
Two positives ions, each carrying a charge q, are separated by a distance d. If F is the force of repulsion between the ions, the number of electrons missing from each ion will be (e being the charge on an electron)
49
A series combination of n1 capacitors, each of value C1, is charged by a source of potential difference 4V. When another parallel combination of n2 capacitors, each of value C2, is charged by a source of potential difference V, it has the same (total) energy stored in it. as the first combination has. The value of C2. in terms of C1, is then
50
The dimension of $${1 \over 2}{\varepsilon _0}{E^2}$$, where $${\varepsilon _0}$$ is permittivity of free space and E is electric field, is