AIPMT 2002
Paper was held on
Sun, May 5, 2002 10:00 AM
Biology
1
What will happen if ligaments are torn?
2
In photosynthersis energy from light reaction to dark reaction is transferred in the form of
3
Which pigment absorbs the red and far-red light?
4
Organisms which obtain energy by the oxidation of reduced inorganic compounds are called
5
How many ATP molecules are produced by aerobic oxidation of one molecule of glucose?
6
Seed dormancy is due to the
7
Dwarfness can be controlled by treating the plant with
8
Impulse of heart beat originates from
9
Which of the following statements is true for lymph?
10
Which of the following absorb light energy for photosynthesis?
11
Which cartilage is present at the end of long bones?
12
Which of the following statement is correct for node of Ranvier of nerve?
13
Mainly which type of hormones control the menstrual cycle in human beings?
14
Acromegaly is caused by
15
When both ovaries are removed from rat then which hormone is decreased in blood?
16
Adrenaline directly affects on
17
In angiosperms pollen tube liberate their male gametes into the
18
In angiosperm all the four microspores of tetrad are covered by a layer which is formed by
19
What is the direction of micropyle in anatropous ovule?
20
What is true for cleavage : -
21
In a population, unrestricted reproductive capacity
is called as -
22
In which of the following animals dimorphic nucleus is found?
23
Which statement is correct for bacterial transduction?
24
The growth curve of bacterial population in lab is plotted against time. What will be the shape of graph?
25
In five kingdom system, the main basis of classification is
26
Some bacteria are able to grow in streptomycin containing medium due to
27
Which of the following secretes toxins during storage conditions of crop plants?
28
In bacteria, plasmid is
29
Which fungal disease spreads by seed and flowers?
30
Choose the correct sequence of stages of growth curve for bacteria.
31
Which of the following is without exception in angiosperms?
32
Which of the following plants produces seeds but not flowers?
33
In which of the following animals nerve cell is present but brain is absent?
34
Which of the following is the example of
pleiotropy ?
35
In which of the following, notochord is present in embryonic stage?
36
In protozoa like Amoeba and Paramecium, the organ for osmoregulation is
37
Geocarpic fruit is
38
Edible part in mango is
39
Which of the following is a reducing sugar?
40
Lipids are insoluble in water because lipid molecules are
41
Mitotic spindle is mainly composed of which protein?
42
Best material for the study of mitosis in laboratory is
43
Wildlife is continuously decreasing.
What is the main reason of this ?
44
Cancerous cells can easily be destroyed by
radiations due to : -
45
If a diploid cell is treated with colchicine then it
becomes : -
46
What is the reason of formation of embryoid
from pollen grain in tissue culture medium ?
47
Which of the following crops have been brought
to India from New world ?
48
Which bacteria is utilized in Gober gas plant ?
49
During the formation of bread it becomes
porous due to release of CO2 by the action of : -
50
Manipulation of DNA in genetic engineering
became possible due to the discovery of : -
51
What is true for individuals of same species ?
52
Which type of association is found in between
entomophilous flower and pollinating agent ?
53
Two different species can not live for long duration
in the same niche or habitat. This law is : -
54
Bamboo plant is growing in a far forest then
what will be the trophic level of it ?
55
In which era reptiles were dominant ?
56
Axillary bud and terminal bud derived from the
activity of : -
57
Four radial vascular bundle are found in : -
58
Vessels are found in : -
59
Which of the following statement is true ?
60
Melanin protect from :-
61
Collagen is : -
62
Ribosomes are produced in :
63
In fluid mosaic model of plasma membrane
64
Which of the following reunites the exon
segments after RNA splicing ?
65
There are three genes a, b and c. The percentage
of crossing over between a and b is 20%, b and c
is 28% and a and c is 8%. What is the sequence
of genes on chromosome
66
On selfing a plant of F1 generation with
genotype “AABbCC”,the genotypic ratio in
F2 generation will be
67
A gene said to be dominant if : -
68
A diseased man marries a normal woman. They
get three daughter and five sons. All the
daughter were diseased and sons were normal.
The gene of this disease is : -
69
Which of the following is a correct match -
70
In a DNA percentage of thymine is 20% then
what is the percentage of guanine : -
71
Transformation experiment was first performed
on which bacteria : -
72
In E. Coli, during lactose metabolism repressor
binds to : -
73
Jacob and Monad studied lactose metabolism in
E.Coli and proposed operon concept. Operon
concept applicable for :
74
Exon part of m-RNAs have code for : -
75
Out of 64 codons, 61 codons code for 20 types
of amino acid it is called : -
76
Which of the following enzymes are used to join
bits of DNA ?
77
Change in sequence of nucleotide in DNA is
called as -
78
Cause of mimicry is -
79
There is no life on moon due to the absence of -
80
Which of the following is important for
speciation ?
81
Genetic drift oparates in : -
82
Which of the following are homologous organs ?
83
Sequence of which of the following is used to
know the phylogeny ?
84
According to fossils which discovered up to
present time, origin and evolution of man was
started from
85
In which condition, the gene ratio remains
constant for any species population ?
Chemistry
1
In the silver plating of copper, K[Ag(CN)2] is used instead of AgNO3. The reason is
2
Which has maximum molecules?
3
In hydrogen atom, energy of first excited state is $$-$$ 3.4 eV. Then find out K.E. of same orbit of hydrogen atom
4
2A $$ \to $$ B + C
It would be a zero order reaction when
It would be a zero order reaction when
5
In the following reaction product $$P$$ is


6

are
7

Product 'P' in the above reaction is
8
Enzymes are made up of
9
Which is not true statement?
10
A solution containing components A and B folloes Raoult's law
11
A solution contains non volatile solute of molecular mass M2. Which of the following can be used to calculate the molecular mass of solute in terms of osmotic pressure?
12
2.5 litre of 1 M NaOH solution is mixed with another 3 litre of 0.5 M NaOH solution. Then find out molarity of resultant solution.
13
In electrolysis of NaCl when Pt electrode is taken then H2 is liberated at cathode while with Hg cathode it forms sodium amalgam
14
Which of the following statement is true?
15
Which of the following order is wrong?
16
General electronic configuration of lanthanides is
17
Which of the following shows maximum number of oxidation states?
18
An atom has electronic configuration 1s2 2s2 2p6 3s2 3p6 3d3 4s2, you will place it in
19
Zn gives H2 gas with H2SO4 and HCl but not with HNO3 because
20
$${}_{92}^{235}$$U, nucleus absorbs a neutron and distintegrate in $${}_{54}^{139}$$Xe, $${}_{38}^{94}$$Sr and $$x$$ so, what will be product $$x$$?
21
Atomic number of Cr and Fe are respectively 24 and 26, which of the following is paramagetic with the spin of electron?
22
The hypothetical complex chloro diaquatriammine cobalt(III) chloride can be represented as
23
CuSO4 when reacts with KCN forms CuCN, which is insoluble in water. It is soluble in excess of KCN, due to formation of the following complex
24
Which has highest pH?
25
Which of the following has p$$\pi $$ $$-$$ d$$\pi $$ bonding?
26
In NO3$$-$$ ion number of bond pair and lone pair of electrons on nitrogen atom are
27
Which of the following is isoelectronic?
28
Unit of entropy is
29
In a closed insulated container a liquid is stirred with a paddle to increase the temperature which of the following is true ?
30
Heat of combustion $$\Delta $$Ho for C(s), H2(g) and CH4(g) are $$-$$ 94, $$-$$ 68 and $$-$$213 kcal/mol, then $$\Delta $$Ho for C(s) + 2H2(g) $$ \to $$ CH4(g) is
31
Which reaction is not feasible?
32
2 mole of ideal gas at 27oC temperature is expanded reversibly from 2 lit. to 20 lit. Find entropy change. (R = 2 cal/mol K)
33
Solubility of MX2 type electrolytes is 0.5 $$ \times $$ 10$$-$$4 mole/lit., then find out Ksp of electrolytes.
34
Reaction BaO2(g) $$\rightleftharpoons$$ BaO(s) + O2(g); $$\Delta $$H = +ve. In equilibrium condition, pressure of O2 depends on
35
Solution of 0.1 N NH4OH and 0.1 N NH4Cl has pH 9.25. Then find out pKb of NH4OH
36
The percentage of C, H and N in an organic compound are 40%, 13.3% and 46.7% respectively then empirical formula is
37
Geometrical isomers differ in
38
IUPAC name of the following is
CH2 $$=$$ CH $$-$$ CH2 $$-$$ CH2 $$-$$ C $$ \equiv $$ CH
CH2 $$=$$ CH $$-$$ CH2 $$-$$ CH2 $$-$$ C $$ \equiv $$ CH
39

In the above reaction product P is
40
When CH3CH2CHCl2 is treated with NaNH2, the product formed is
41
Reactivity order of halides for dehydrologenation is
42

Z in the above reaction sequence is
43
n-propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent
44
When phenol is treated with CHCl3 and NaOH, the product formed is
Physics
1
A capacitor of capacity C1 charged upto V volt and then connected to an uncharged capacitor of capacity C2. The final potential difference across each will be
2
When ultraviolet rays incident on metal plate then photoelectric effect does not occur, it occurs by incidence of
3
To convert a galvanometer into a voltmeter one should connect a
4
A charge q moves in a region where electric field and magnetic field both exist, then force on it is
5
The magnetic field of given length of wire for single turn coil at its centre is B then its value for two turns coil for the same wire is
6
Two bar magnets having same geometry with magnetic moments M and 2M, are firstly placed in such a way that their similar poles are same side then its time period of oscillation is T1. Now the polarity of one of the magnet is reversed then time period of oscillation is T2, then
7
For a series LCR circuit the power loss at resonance is
8
The velocity of electromagnetic wave is parallel to
9
What is the cause of Green house effect ?
10
A bulb is located on a wall. Its image is to be obtained on a parallel wall with the help of convex lens. The lens is placed at a distance d ahead of second wall, then required focal length will be
11
Diameter of human eye lens is 2 mm. What will be the minimum distance between two points to resolve them, which are situated at a distance of 50 meter from eye. The wavelength of light is 5000 $$\mathop A\limits^ \circ $$
12
For the given incident ray as shown in figure, the condition of total internal refraction of this ray the required refractive index of prism will be


13
Which of the following is not the property of cathode rays ?
14
Specific resistance of a conductor increases with
15
If particles are moving with same velocity, then which has maximum de Broglie wavelength?
16
The value of Planck's constant is
17
Which of the following are suitable for the fusion process ?
18
A deutron is bombarded on 8O16 nucleus then $$\alpha $$-particle is emitted. The product nucleus is
19
Number of atom per unit cell in B.C.C.
20
In a p-n junction
21
The given truth table is for which logic gate
$$\matrix{ A & B & Y \cr 1 & 1 & 0 \cr 0 & 1 & 1 \cr 1 & 0 & 1 \cr 0 & 0 & 1 \cr } $$
$$\matrix{ A & B & Y \cr 1 & 1 & 0 \cr 0 & 1 & 1 \cr 1 & 0 & 1 \cr 0 & 0 & 1 \cr } $$
22
For the given circuit of p-n junction diode which is correct


23
Consider two rods of same length and different specific heats (S1, S2), conductivities (K1, K2) and area of cross-sections (A1, A2) and both having temperatures T1 and T2 at their ends. If rate of loss of heat due to conduction is equal, then
24
An object of mass 3 kg is at rest. Now a force of $$\overrightarrow F $$ = 6t2 $$\widehat i$$ + 4t $$\widehat j$$ is applied on the object then velocity of object at t = 3 sec. is
25
A lift of mass 1000 kg which is moving with acceleration of 1 m/s2 in upward direction, then the tension developed in string which is connected to lift is
26
A block of mass 10 kg placed on rough horizontal surface having coefficient of friction m = 0.5, if a horizontal force of 100 N acting on it then acceleration of the block will be
27
If kinetic energy of a body is increased by 300% then percentage change in momentum will be :
28
A rod of length is 3 m and its mass acting per unit length is directly proportional to distance x from one of its end then its centre of gravity from that end will be at :
29
A point P consider at contact point of a wheel on ground which rolls on ground without slipping then value of displacement of point P when wheel completes half of rotation (if radius of wheel is 1 m)
30
A solid sphere of radius R is placed on smooth horizontal surface. A horizontal force F is applied at height h from the lowest point. For the maximum acceleration of centre of mass, which is correct ?
31
A disc is rotating with angular speed $$\omega $$. If a child sits on it, what is conserved
32
A circular disc is to be made by using iron and aluminium so that it acquired maximum moment of inertia about geometrical axis. It is possible with
33
Unit of Stefan's constant is
34
For a black body at temperature 727oC, its radiating power is 60 watt and temperature of surrounding is 227oC. If temperature of black body is changed to 1227oC then its radiating power will be
35
Which of the following is best close to an ideal black body?
36
The Wien's displacement law express relation between
37
The efficiency of Carnot engine is 50% and temperature of sink is 500 K. If temperature of source is kept constant and its efficiency raised to 60%, then the required temperature of sink will be
38
Displacement between maximum potential energy position and maximum kinetic energy position for a particle executing simple harmonic motion is
39
When an oscillator completes 100 oscillations its amplitude reduced to $${1 \over 3}$$ of initial value. What will be its amplitude, when it complettes 200 oscillations ?
40
A mass is suspended separately by two different springs in (successive order then time periods is t1 and t2 respectively, If it is connected by both spring as shown in figure then time period is t0 , the correct relation is
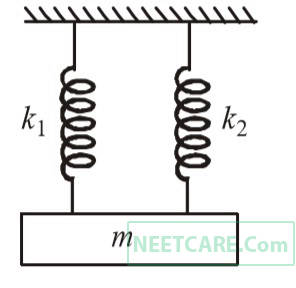

41
A wave travelling in positive X-direction with a $$=$$ 0.2 ms$$-$$2, velocity = 360 ms$$-$$1 and $$\lambda $$ $$=$$ 60 m, then correct expression for the wave is
42
Identical charges ($$-$$q) are placed at each corners of cube of side b then electrostatic potential energy of charge (+q) which is placed at centre of cube will be
43
Some charge is being given to a conductor. Then its potential is
44
For a cell terminal potential difference is 2.2V when circuit is open and reduce to 1.8 V when cell is connected to a resistance of R = 5 $$\Omega $$. Determine internal resistance of cell (r)
45
A particle A is dropped from a height and another particle B is projected in horizontal direction with speed of 5/sec from the same height then correct statement is