AIPMT 2006
Paper was held on
Sun, May 7, 2006 10:00 AM
Biology
1
The contractile protein of skeletal muscle involving ATPase activity is
2
In photosystem I, the first electron acceptor is
3
How many ATP molecules could maximally be generated from one molecule of glucose, if the complete oxidation of one mole of glucose to CO2 and H2O yields 686 kcal and the useful chemical energy available in the high energy phosphate bond of one mole of ATP is 12 kcal?
4
Farmers in a particular region were concerned that pre-mature yellowing of leaves of a pulse crop might cause decrease in the yield. Which treatment could be most beneficial to obtain maximum seed yield?
5
An enzyme that can stimulate germination of barley seeds is
6
How does pruning help in making the hedge dense?
7
Treatment of seeds at low temperature under moist conditions to break its dormancy is called
8
The majority of carbon dioxide produced by our body cells is transported to the lungs as
9
Which one of the following statements is incorrect?
10
People living at sea level have around 5 million RBC per cubic millimeter of their blood whereas those living at altitude to 5400 metres have around 8 million. This is because at high altitude
11
Which one of the following has an open circulatory system?
12
Angiotensinogen is a protein produced and secreted by
13
During photorespiration, the oxygen consuming reaction(s) occur in
14
Bowman's glands are found in
15
Which one of the following does not act as a neurotransmitter?
16
Which hormone causes dilation of blood vessels, increased oxygen consumption and glucogenesis?
17
Which of the following is an accumulation and release center of neurohormones?
18
A steroid hormone which regulates glucose metabolism is
19
Which one of the following statements is correct?
20
Which one of the following is not a secondary messenger in hormone action?
21
Parthenocarpic tomato fruits can be produced by
22
In a cereal grain the single cotyledon of embryo is represented by
23
The arrangement of the nuclei in a normal embryo sac in the dicot plants is
24
Sertoli cells are regulated by the pituitary
hormone known as-
25
Which one of the following is not a living fossil?
26
Curing of tea leaves is brought about by the activity of
27
Which of the following environmental conditions are essential for optimum growth of Mucor on a piece of bread?
A. Temperature of about 25o C
B. Temperature of about 5o C
C. Relative humidity of about 5%
D. Relative humidity of about 95%
E. A shady place
F. A brightly illuminated place
Choose the answer from the following options.
A. Temperature of about 25o C
B. Temperature of about 5o C
C. Relative humidity of about 5%
D. Relative humidity of about 95%
E. A shady place
F. A brightly illuminated place
Choose the answer from the following options.
28
Peat moss is used as a packing material for sending flowers and live plants to distant places because
29
Conifers differ from grasses in the
30
In a moss, the sporophyte
31
Two common characters found in centipede, cockroach, and crab are
32
Biradial symmetry and lack of cnidoblasts are the characteristic of
33
In which one of the following sets of animals do all the four give birth to young ones?
34
Withdrawal of which of the following hormones
is the immediate cause of menstruation ?
35
Annual migration does not occur in the case of
36
Which one of the following is a matching set of a phylum and its three examples?
37
Metameric segmentation is the characteristic of
38
What is common about Trypanosoma, Noctiluca, Monocystis and Giardia?
39
Pentamerous actinomorphic flowers, bicarpellary ovary with oblique septa, and fruit capsule or berry, are characteristic features of
40
In which of the following fruits, the edible part is the aril ?
41
Pineapple (ananas) fruit develops from
42
Long filamentous threads protruding at the end of a young cob of maize are
43
An organic substance bound to an enzyme and essential for its activity is called
44
Which one of the following is not included
under in-situ conservation ?
45
Restriction endonuclease -
46
Two microbes found to be very useful in
genetic engineering are-
47
Golden rice is a promising transgenic crop.
When released for cultivation, it will help in
48
Niche overlap indicates-
49
Which one of the following is not used for
construction of ecological pyramids ?
50
Which of the following pairs of an animal and a
plant represents endangered organisms in India ?
51
Which of the following is considered a hot-spot
of biodiversity in India ?
52
Antibodies in our body are complex-
53
Which one of the following is the correctly
matched pair of an endangered animal and a
National Park ?
54
A common structural feature of vessel elements
and sieve tube elements is-
55
Areolar connective tissue joins-
56
Mast cells secrete-
57
Earthworms are -
58
Which of the following statements regarding
mitochondrial membrane is not correct ?
59
Which of the following statements regarding
cilia is not correct ?
60
A major breakthrough in the studies of cells
came with the development of electron
microscope. This is because
61
One turn of the helix in a B-form DNA is
approximately -
62
The formula for exponential population growth is-
63
Test cross involves-
64
Both sickle cell anemia and Huntington's chorea
are-
65
If a colour blind woman marries a normal visioned
man, their sons will be
66
Cri-du-chat syndrome in humans is caused by
the-
67
In Mendel's experiments with garden pea, round
seed shape (RR) was dominant over wrinkled
seeds (rr), yellow cotyledon (YY) was dominant
over green cotyledon (yy). What are the
expected phenotypes in the F2 generation of the
cross RRYY × rryy ?
68
Sickle cell anaemia has not been eliminated from
the African population because-
69
How many different kinds of gametes will be
produced by a plant having the genotype
AABbCC ?
70
Which one of the following is an example of
polygenic inheritance ?
71
Phenotype of an organism is the result of-
72
One gene-one enzyme hypothesis was
postulated by -
73
Which antibiotic inhibits interaction between
tRNA and mRNA during bacterial protein
synthesis ?
74
Antiparallel strands of a DNA molecule means
that-
75
Amino acid sequence, in protein synthesis is
decided by the sequence of -
76
Which one of the following amino-acids was not
found to be synthesized in Miller's experiment ?
77
An important evidence in favour of organic
evolution is the occurrence of-
78
Praying mantis is a good example of-
79
Jurassic period of the Mesozoic era
characterized by-
80
The bacterium (Clostridium botulinum) that
causes botulism is-
81
The causative agent of mad-cow disease is a-
82
A person showing unpredictable moods,
outbursts of emotion, quarrelsome behaviour
and conflicts with others is suffering from-
83
HIV that causes AIDS, first starts destroying
Chemistry
1
Copper sulphate dissolves in excess of KCN to give
2
[Cr(H2O)6]Cl3 (At. no. of Cr = 24) has a magnetic momen of 3.83 B.M. The correct distribution of 3d electrons in the chromium of the complex is
3
[Co(NH3)4(NO2)2]Cl exhibits
4
More number of oxidation states are exhibited by the actinoids than by the lanthanoids. The main reason for this is
5
In a set of reactions propionic acid yielded a compound D.

The structure of D would be

The structure of D would be
6
Which of the following is more basic than aniline?
7
The human body does not produce
8
Which one of the following is a peptide hormone?
9
During the process of digestion, the proteins present in food materials are hydrolysed to amino acids. The two enzymes involved in the process-

are respectively

are respectively
10
During osmosis, flow of water through a semipermeable membrane is
11
A solution of acetone in ethanol
12
1.00 g of a non-electrolyte solute (molar mass 250 g mol$$-$$1) was dissolved in 51.2 g of benzene. If the freezing point depression constant, Kf of benzene is 5.12 K kg mol$$-$$1, the freezing point of benzene will be lowered by
13
A solution containing 10 g per dm3 of urea (molecular mass = 60 g mol$$-$$1) is isotonic with a 5% solution of a nonvolatile solute is
14
Self condensation of two moles of ethyl acetate in presence of sodium ethoxide yields
15
EoFe2+/Fe = $$-$$ 0.441 V and EoFe3+/Fe2+ = 0.771 V, the standard EMF of the reaction Fe + 2Fe3+ $$ \to $$ 3Fe2+ will be
16
A hypothetical electrochemical cell is shown below.
$$A\left| {{A^ + }\left( {xM} \right)} \right|\left| {{B^ + }\left( {yM} \right)} \right|B$$
The emf measured is + 0.20 V. The cell reaction is
$$A\left| {{A^ + }\left( {xM} \right)} \right|\left| {{B^ + }\left( {yM} \right)} \right|B$$
The emf measured is + 0.20 V. The cell reaction is
17
Consider the reaction : N2(g) + 3H2(g) $$ \to $$ 2NH3(g)
The equality relationship between $${{d\left[ {N{H_3}} \right]} \over {dt}}$$ and $$ - {{d\left[ {{H_2}} \right]} \over {dt}}$$ is
The equality relationship between $${{d\left[ {N{H_3}} \right]} \over {dt}}$$ and $$ - {{d\left[ {{H_2}} \right]} \over {dt}}$$ is
18
For the reaction, 2A + B $$ \to $$ 3C + D, which of the following does not express the reaction rate?
19
Which of the following is the most basic oxide?
20
The correct order regarding the electronegativity of hybrid orbitals of carbon is
21
In which of the following molecules are all the bonds are not equal?
22
Which one of the following orders is not in accordance with the property stated against it?
23
The electronegativity difference berween N and F is greater than that between N and H yet the dipole moment of NH3 (1.5D) is larger than that of NF3 (0.2 D). This is because
24
In which of the following pairs are both the ions coloured in aqueous solution?
(At. no. : Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)
(At. no. : Sc = 21, Ti = 22, Ni = 28, Cu = 29, Co = 27)
25
For the reaction :
CH4(g) + 2O2(g) $$\rightleftharpoons$$ CO2(g) + 2H2O(l),
$$\Delta $$Hr = $$-$$ 170.8 kJ mol$$-$$1.
Which of the following statements is not true?
CH4(g) + 2O2(g) $$\rightleftharpoons$$ CO2(g) + 2H2O(l),
$$\Delta $$Hr = $$-$$ 170.8 kJ mol$$-$$1.
Which of the following statements is not true?
26
The orientation of an atomic orbital is governed by
27
Given : The mass of electron is 9.11 $$ \times $$ 10$$-$$31 kg, Planck constant is 6.626 $$ \times $$ 10$$-$$34 J s, the uncertainty involved in the measurement of velocity within a distance of 0.1 $$\mathop A\limits^ \circ $$ is
28
Which one of the following orders is not in accordance with the property stated against it ?
29
Which of the following is not a correct statement?
30
Which of the following is not isostructural with SiCl4 ?
31
Which of the following species has a linear shape?
32
Identify the correct statement for change of Gibb's energy for a system ($$\Delta $$Gsystem) at constant temperature and pressure.
33
The enthalpy and entropy change for the reaction:
Br2(l) + Cl2(g) $$ \to $$ 2BrCl(g)
are 30 kJ mol$$-$$1 and 105 J K$$-$$1 mol$$-$$1 respectively.
The temperature at which the reaction will be in equilibrium is
Br2(l) + Cl2(g) $$ \to $$ 2BrCl(g)
are 30 kJ mol$$-$$1 and 105 J K$$-$$1 mol$$-$$1 respectively.
The temperature at which the reaction will be in equilibrium is
34
The enthalpy of hydrogenation of cyclohexene is is $$-$$ 119.5 kJ mol$$-$$1. If resonance energy of benzene is $$-$$ 150.4 kJ mol$$-$$1, its enthalpy of hydrogenation would be
35
Assume each reaction is carried out in an open container. For which reaction will $$\Delta $$H = $$\Delta $$E ?
36
Which of the following pairs constitutes a buffer?
37
The hydrogen ion concentration of a 10$$-$$8 M, HCl aqueous solution at 298 K (Kw = 10$$-$$14) is
38
The general molecular formula, which represents the homologous series of alkanols is
39
The IUPAC name of

is

is
40
Which of the following is not chiral?
41
The general molecular formula, which represents the homologous series of alkanols is
42
Ethylene oxide when treated with Grignard reagent yields
43
The major organic product in the reaction is
CH3 $$-$$ O $$-$$ CH(CH3)2 + HI $$ \to $$ products
CH3 $$-$$ O $$-$$ CH(CH3)2 + HI $$ \to $$ products
44
A carbonyl compound reacts with hydrogen cyanide to form cyanohydrin which on hydrolysis forms a racemic mixture of $$\alpha $$-hydrixy acid. The carbonyl compound is
45
Nucleophilic addition reaction will be most favoured in
Physics
1
A transistor-oscillator using a resonant circuit with an inductor L (of negligible resistance) and a capacitor C in series produce oscillations of frequency $$f$$. If L is doubled and C is changed to 4C, the frequency will be
2
An electric dipole of moment $$\overrightarrow p $$ is lying along a uniform electric field $$\overrightarrow E $$. The work done in rotating the dipole by 90o is
3
In the circuit shown, if a conducting wire is connected between points A and B, the current in this wire will
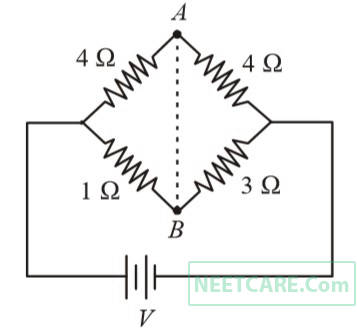

4
Kirchoff's first and second laws of electrical circuits are consequences of
5
Two cells, having the same e.m.f. are connected in series through an external resistance R. Cells have internal resistances r1 and r2 (r1 > r2) respectively. When the circuit is closed, the potential difference across the first cell is zero. The value of R is
6
Power dissipated across the 8 $$\Omega $$ resistor in the circuit shown here is 2 watt. The power dissipated in watt units across the 3 $$\Omega $$ resistor is
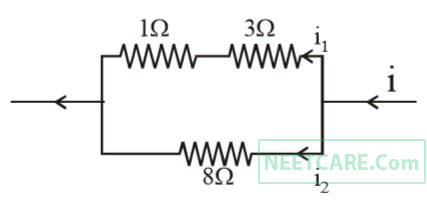

7
When a charged particle moving with velocity $$\overrightarrow v $$ is subjected to a magnetic field of induction $$\overrightarrow B $$, the force on it is non-zero. This implies that
8
Two circular coils 1 and 2 are made from the same wire but the radius of the 1st coil is twice that of the 2nd coil. What is the ratio of potential difference in volts should be applied across them so that the magnetic field at their centres is the same?
9
Curie temperature above which
10
Two coils of self inductance 2 mH and 8 mH are placed so close together that the effective flux in one coil is completely linked with the other. The mutual inductance between these coils is
11
A coil of inductive reactance 31 $$\Omega $$ has a resistance of 8 $$\Omega $$. It is placed in series with a condenser of capacitative reactance 25 $$\Omega $$. The combination is connected to an a.c. source of 110 V. The power factor of the circuit is
12
The core of a transformer is laminated because
13
A square surface of side L metres is in the plane of the paper. A uniform electric field $$\overrightarrow E $$ (volt/m), also in the plane of the paper is limited only to the lower half of the square surface (see figure). The electric flux in SI inits associated with the surface is


14
A microscope is focussed on a mark on a piece of paper and then a slab of glass of thickness 3 cm and refractive index 1.5 is placed over the mark. How should the microscope be moved to get the mark in focus again ?
15
A convex lens and a concave lens, each having same focal length of 25 cm, are put in contact to form a combination of lenses. The power in diopters of the combination is
16
When photons of energy h$$\upsilon $$ fall on an aluminimum plate (of work function E0), photoelectrons of maximum kinetic energy K are ejected. If the frequency of radiation is doubled, the maximum kinetic energy of the ejected photoelectrons will be
17
A photocell employs photoelectric effect to convert
18
The momentum of a photon of energy 1 MeV in kg m/s will be
19
In a discharge tube ionization of enclosed gas is produced due to collisions between
20
Ionization potential of hydrogen atom is 13.6 eV. Hydrogen atoms in the ground state are excited by monochromatic radiation of photon energy 12.1 eV. According to Bohr's theory, the spectral lines emited by hydrogen will be
21
The binding energy of deuteron is 2.2 MeV and that of $${}_2^4$$He is 28 MeV. If two deuterons are fused to form one $${}_2^4$$He then the energy released is
22
The following figure shows a logic gate circuit with two inputs A and B and the output C.

The voltage waveforms of A, B and C are as shown below.

The logic circuit gate is

The voltage waveforms of A, B and C are as shown below.

The logic circuit gate is
23
A tube of length L is filled completely with an incompressible liquid of mass M and closed at both the ends. The tube is then rotated in a horizontal plane about one of its ends with a uniform angular velocity $$\omega $$. The force exerted by the liquid at the other end is
24
Two bodies A (of mass 1 kg) and B (of mass 3 kg) are dropped from heights of 16 m and 25 m, respectively. The ratio of the time taken by them to reach the ground is
25
A car runs at a constant speed on a circular track of radius 100 m, taking 62.8 seconds for every circular lap. The average velocity and average speed for each circular lap respectively is
26
A particle moves along a straight line OX. At a time t (in seconds) the distance x (in metres) of the particle from O is given by x = 40 + 12t $$-$$ t3. How long would the particle travel before coming to rest ?
27
For angles of projection of a projectile at angle (45o $$-$$ $$\theta $$) and (45o + $$\theta $$), the horizontal range described by the projectile are in the ratio of
28
The vectors $$\overrightarrow A $$ and $$\overrightarrow B $$ are such that $$\left| {\overrightarrow A + \overrightarrow B } \right| = \left| {\overrightarrow A - \overrightarrow B } \right|.$$ The angle between the two vectors is
29
A 0.5 kg ball moving with a speed of 12 m/s strikes a hand wall at an angle of 30o with the wall. It is reflected with the same speed at the same angle. If the ball is in contact with the wall for 0.25 seconds, the average force acting on the wall is :
30
300 J of work is done in sliding a 2 kg block up an inclined plane of height 10 m. Work done against friction is (Take g = 10 m/s2)
31
A body of mass 3 kg is under a constant force which causes a displacement s in metres in it, given by the relation s = $${1 \over 3}$$t2, where t is in seconds. Work done by the force in 2 seconds is
32
The potential energy of a long spring when stretched by 2 cm is U. If the spring is stretched by 8 cm the potential energy stored in it is
33
A uniform rod AB of length $$l$$ and mass m is free to rotate about point A. The rod is released from rest in the horizontal position. Given that the moment of inertia of the rod about A is ml2/3, the initial angular acceleration of the rod will be
34
The moment of inertia of a uniform circular disc of radius R and mass M about an axis touching the disc at its diameter and normal to the disc
35
The earth is assumed to be a sphere of radius R. A platform is arranged at a height R from the surface of the earth. The escape velocity of a body from this platform is fv, where v is its escape velocity from the surface of the Earth. The value of f is
36
A black body at 1227oC emits radiations with maximum intensity at a wavelength of 5000 $$\mathop A\limits^ \circ $$. If the temperature of the body is increased by 1000oC, the maximum intensity will be observed at
37
The molar specific heat at constant pressure of an ideal gas is (7/2) R. The ratio of specific heat at constant pressure to that at constant volume is
38
A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of source be increased so as to increase its efficiency by 50% of original efficiency ?
39
A rectangular block of mass m and area of cross-section A floats in a liquid of density $$\rho $$. If it is given a small vertical displacement from equilibrium it undergoes with a time period T, then
40
Two vibrating tuning forks produce waves given by y1 = 4 sin 500$$\pi $$t and y2 = 2 sin506 $$\pi $$t. Number of beats produced per minute is
41
Two sound waves with wavelengths 5.0 m and 5.5. m respectively, each propagate in a gas with velocity 330 m/s. We expect the following number of beats per second.
42
The time of reverberation of a room A is one second. What will be the time (in seconds of reverberation of a room, having all the dimensions double of those of room A ?
43
A transverse wave propagating along x-axis is represented by y(x, t) = 8.0 sin (0.5 $$\pi $$x $$-$$ 4$$\pi $$t $$-$$ $$\pi $$/4) where x is in metres and t is in seconds. The speed of the wave is
44
Which one of the following statements is true ?
45
A parallel plate air capacitor is charged to a potential difference of V volts. After disconnecting the charging battery the distance between the plates of the capacitor is increased using an insulting handle. As a result the potential difference between the plates
46
Which one of the following represents forward bias diode ?
47
The velocity v of a particle at time t is given by v = at + $${b \over {t + c}}$$, where a, b and c are constants. The dimensions of a, b and c are