AIPMT 2007
Paper was held on
Sun, May 6, 2007 10:00 AM
Biology
1
Opening of floral buds into flowers, is a type of
2
Which of the following is a flowering plant with nodules containing filamentous nitrogen-fixing micro-organism?
3
About 98 percent of the mas of every living organism is composed of just six elements including carbon, hydrogen, nitrogen, oxygen and
4
The first acceptor of electrons from an excited chlorophyll molecule of photosystem II is
5
In the leaves of C4 plants, malic acid formation during CO2 fixation occurs in the cells of
6
All enzymes of TCA cycle are located in the mitochondrial matrix except one which is located in inner mitochondrial membranes in eukaryotes and in cytosol in prokaryotes. This enzyme is
7
The overall goal of glycolysis, Krebs' cycle and the electron transport system is the formation of
8
The wavelength of light absorbed by Pr form of phytochrome is
9
What is common between parrot, platypus and kangaroo?
10
Which one of the following pairs, is not correctly matched?
11
A drop of each of the following, is placed separately on four slides. Which of them will not coagulate?
12
A person who is on a long hunger strike and is surviving only on water, will have
13
In human body, which one of the following is anatomically correct?
14
Which one of the following items gives its correct total number?
15
During the transmission of nerve impulse through a nerve fibre, the potential on the inner side of the plasma membrane has which type of electric change?
16
Bowman's glands are located in the
17
Feeling the tremors of an earthquake a scared resident of seventh floor of a multistoryed building starts climbing down the stairs rapidly. Which hormone initiated this action?
18
A person is having problems with calcium and phosphorus metabolism in his body. Which one of the following glands may not be functioning properly?
19
Which one of the following is a slime mould?
20
ICBN stands for
21
The living organisms can be unexceptionally distinguished from the non-living things on the basis of their ability for
22
Two plants can be conclusively said to belong to the same species if they
23
Which one of the following statements about mycoplasma is wrong?
24
Which pair of the following belongs to basidiomycetes?
25
In gymnosperms, the pollen chamber represents
26
Spore dissemination in some liverworts is added by
27
In the prothallus of a vascular cryptogam, the antherozoids and eggs mature at different times. As a result
28
If you are asked to classify the various algae into distinct groups, which of the following characters you should choose?
29
Flagellated male gametes are present in all the three of which one of the following sets?
30
Which one of the following is a matching pair of body feature and the animal possessing it?
31
Which of the following pairs are correctly matched?
| Animals | Morphological features | ||
|---|---|---|---|
| (i) | Crocodile | - | 4-chambered heart |
| (ii) | Sea urchin | - | Parapodia |
| (iii) | Obelia | - | Metagenesis |
| (iv) | Lemur | - | Thecodont |
32
What is true about Nereis, scorpion, cockroach and silver fish?
33
A genetically engineered micro-organism used
successfully in bioremediation of oil spills is a
species of-
34
Geometric representation of age structure is a characteristic of :-
35
The population of an insect species shows an
explosive increase in numbers during rainy
season followed by its disappearance at the end
of the season. What does this show ?
36
If the mean and the median pertaining to a
certain character of a population are of the same
value, the following is most likely to occur:-
37
A high density of elephant population in an area
can result in :-
38
Which one of the following ecosystem types has
the highest annual net primary productivity ?
39
Which one of the following pairs of organisms
are exotic species introduced in India ?
40
Identify the odd combination of the habitat and
the particular animal concerned :
41
One of endangered species of Indian medicinal
plants is that of:-
42
Probiotics are-
43
Passage cells are thin-walled cells found in:-
44
For a critical study of secondary growth in plants,
which one of the following pairs is suitable ?
45
Which one of the following pairs of structures
distinguishes a nerve cell from other types of cell ?
46
In which one of the following preparations are
you likely to come across cell junctions most
frequently ?
47
Which one of the following is not a constituent
of cell membrane ?
48
Biological organization starts with:-
49
Select the wrong statement from the following:
50
Which one of the following statements is correct?
51
When two species of different genealogy come
to resemble each other as a result of adaptation,
the phenomenon is termed:-
52
Male gametes in angiosperms are formed by the division of
53
Which one of the following is surrounded by a callose wall?
54
Which part of ovary in mammals acts as an
endocrine gland after ovulation ?
55
In the human female, menstruation can be
deferred by the administration of:-
56
A human male produces sperms with the
genotypes AB, Ab, AB, and ab pertaining to two
diallelic characters in equal proportions. What is
the corresponding genotype of this person ?
57
In pea plants, yellow seeds are dominant to
green. If a heterozygous yellow seeded plant is
crossed with a green seeded plant, what ratio of
yellow and green seeded plants would you
expect in F1 generation ?
58
Inheritance of skin colour in humans is an
example of:-
59
A common test to find the genotype of a hybrid
is by:-
60
In the hexaploid wheat, the haploid(n) and
basic(x) numbers of chromosomes are :
61
The concept of chemical evolution is based on-
62
Adaptive radiation refers to:-
63
The finches of Galapogas islands provide an
evidence in favour of-
64
If you suspect major deficiency of antibodies in
a person, to which of the following would you
look for confirmatory evidence?
65
Lysozyme that is present in perspiration, saliva
and tears, destroys:
66
Increased asthmatic attacks in certain seasons are
related to:
67
Which one of the following pairs is wrongly
matched ?
Chemistry
1
Which of the following anions is present in the chain structure of silicates?
2
Which one of the following orders correctly represents the increasing acid strengths of the given acids?
3
Which one of the following ions is the most stable in aqueous solution?
(At. No. Ti = 22, V = 23, Cr = 24, Mn = 25)
(At. No. Ti = 22, V = 23, Cr = 24, Mn = 25)
4
Identify the incorrect statement among the following:
5
Which of the following will give a pair of enantiomorphs?
(en = NH2CH2CH2NH2)
(en = NH2CH2CH2NH2)
6
The d electron configurations of Cr2+, Mn2+, Fe2+ and Ni2+ are 3d4, 3d5, 3d6 and 3d8 respectively. Which one of the following aqua complexes will exhibit the minimum paramagnetic behaviour?
(At. No. Cr = 24, Mn = 25, Fe = 26, Ni = 28)
(At. No. Cr = 24, Mn = 25, Fe = 26, Ni = 28)
7
Which of the following oxidation states are the most characteristic for lead and tin respectively?
8
Reduction of aldehydes and ketones into hydrocarbons using zinc amalgam and conc. HCl is called
9
The product formed in Aldol condensation is
10
Consider the following compounds

The correct decreasing order of their reactivity towards hydrolysis is

The correct decreasing order of their reactivity towards hydrolysis is
11
Which of the following represents the correct order of the acidity in the given compounds ?
12
Which one of the following on treatment with 50% aqueous sodium hydroxide yields the corresponding alcohol and acid?
13
Which one of the following on reduction with lithium aluminium hydride yields a secondary amine?
14
Which of the following vitamins is water-soluble?
15
RNA and DNA are chiral molecules, their chirality is due to
16
In the reaction :

Which of the following compounds will be formed?

Which of the following compounds will be formed?
17
Concentrated aqueous sulphuric acid is 98% H2SO4 by mass and has a density of 1.80 g mL$$-$$1. Volume of acid required to make one litre of 0.1 M H2SO4 solution is
18
0.5 molal aqueous solution of a weak acid (HX) is 20% ionised. If Kf for water is 1.86 K kg mol$$-$$1, the lowering in freezing point of the solution is
19
The equilibrium constant of the reaction:
Cu(s) + 2Ag+(aq) $$ \to $$ Cu2+(aq) + 2Ag(s);
Eo = 0.46 V at 298 K is
Cu(s) + 2Ag+(aq) $$ \to $$ Cu2+(aq) + 2Ag(s);
Eo = 0.46 V at 298 K is
20
The efficiency of a fuel cell is given by
21
If 60% of a first order reaction was completed in 60 minutes, 50% of the same reaction would be completed in approximately
(log 4 = 0.60, log 5 = 0.69)
(log 4 = 0.60, log 5 = 0.69)
22
In a first-order reaction A $$ \to $$ B, if k is rate constant and initial concentration of the reactant A is 0.5 M, then the half-life is
23
The reaction of hydrogen and iodine monochloride is given as :
H2(g) + 2ICl(g) $$ \to $$ 2HCl(g) + I2(g)
This reaction is of first order with respect to H2(g) and ICl(g),
following mechanisms were proposed.
Mechanism A :
H2(g) + 2ICl(g) $$ \to $$ 2HCl(g) + I2(g)
Mechanism B :
H2(g) + ICl(g) $$ \to $$ HCl(g) + HI(g) ; slow
HI(g) + ICl(g) $$ \to $$ HCl(g) + I2(g) ; fast
Which of the above mechanism(s) can be consistent with the given information about the reaction?
H2(g) + 2ICl(g) $$ \to $$ 2HCl(g) + I2(g)
This reaction is of first order with respect to H2(g) and ICl(g),
following mechanisms were proposed.
Mechanism A :
H2(g) + 2ICl(g) $$ \to $$ 2HCl(g) + I2(g)
Mechanism B :
H2(g) + ICl(g) $$ \to $$ HCl(g) + HI(g) ; slow
HI(g) + ICl(g) $$ \to $$ HCl(g) + I2(g) ; fast
Which of the above mechanism(s) can be consistent with the given information about the reaction?
24
Consider the following reactions :
(i) H+(aq) + OH$$-$$(aq) = H2O(l), $$\Delta $$H = $$-$$ X1 kJ mol$$-$$1
(ii) H2(g) + 1/2O2(g) = H2O(l), $$\Delta $$H = $$-$$ X2 kJ mol$$-$$1
(iii) CO2(g) + H2(g) = CO(g) + H2O(l), $$\Delta $$H = $$-$$ X3 kJ mol$$-$$1
(iv) C2H2(g) + 5/2O2(g) = 2CO2(g) + H2O(l), $$\Delta $$H = +X4 kJ mol$$-$$1
Enthalpy of formation of H2O(l) is
(i) H+(aq) + OH$$-$$(aq) = H2O(l), $$\Delta $$H = $$-$$ X1 kJ mol$$-$$1
(ii) H2(g) + 1/2O2(g) = H2O(l), $$\Delta $$H = $$-$$ X2 kJ mol$$-$$1
(iii) CO2(g) + H2(g) = CO(g) + H2O(l), $$\Delta $$H = $$-$$ X3 kJ mol$$-$$1
(iv) C2H2(g) + 5/2O2(g) = 2CO2(g) + H2O(l), $$\Delta $$H = +X4 kJ mol$$-$$1
Enthalpy of formation of H2O(l) is
25
An element, X has the following isotopic composition:
200X : 90% 199X : 8.0% 202X : 2.0%
The weighted average atomic mass of the naturally occuring element X is closest to
200X : 90% 199X : 8.0% 202X : 2.0%
The weighted average atomic mass of the naturally occuring element X is closest to
26
Consider the following sets of quantum numbers :
Which of the following sets of quantum number is not possible ?
| n | l | m | s | ||||
|---|---|---|---|---|---|---|---|
| (i) | 3 | 0 | 0 | +1/2 | |||
| (ii) | 2 | 2 | 1 | +1/2 | |||
| (iii) | 4 | 3 | -2 | -1/2 | |||
| (iv) | 1 | 0 | -1 | -1/2 | |||
| (v) | 3 | 2 | 3 | +1/2 |
Which of the following sets of quantum number is not possible ?
27
Which one of the following ionic species has the greatest proton affinity to form stable compound ?
28
Identify the correct order of the size of the following :
29
With which of the following electronic configuration an atom has the lowest ionisation enthalpy ?
30
In which of the following pairs, the two species are isostructural?
31
The correct order of C $$-$$ O bond length among CO, CO$${_3^{2 - }}$$, CO2 is
32
Given that bond energies of H $$-$$ H and Cl $$-$$ Cl are 430 kJ mol$$-$$1 and 240 kJ mol$$-$$1 respectively and $$\Delta $$Hf for HCl is $$-$$ 90 kJ mol$$-$$1, bond enthalpy of HCl is
33
The equilibrium constants of the following are
N2 + 3H2 $$\rightleftharpoons$$ 2NH3; K1
N2 + O2 $$\rightleftharpoons$$ 2NO; K2
H2 + $${1 \over 2}$$O2 $$\rightleftharpoons$$ H2O; K3
The equilibrium constant (K) of the reaction :
2NH3 + $${5 \over 2}$$ O2 $$\rightleftharpoons$$ 2NO + 3H2O will be
N2 + 3H2 $$\rightleftharpoons$$ 2NH3; K1
N2 + O2 $$\rightleftharpoons$$ 2NO; K2
H2 + $${1 \over 2}$$O2 $$\rightleftharpoons$$ H2O; K3
The equilibrium constant (K) of the reaction :
2NH3 + $${5 \over 2}$$ O2 $$\rightleftharpoons$$ 2NO + 3H2O will be
34
A weak acid, HA, has a Ka of 1.00 $$ \times $$ 10$$-$$5. If 0.100 mol of this acid is dissolved in one litre of water, the percentage of acid dissociated at equilibrium is closest to
35
Calculate the pOH of a solution at 25oC that contains 1 $$ \times $$ 10$$-$$10 M of hydronium ions, i.e. H3O+.
36
CH3 $$-$$ CHCl $$-$$ CH2 $$-$$ CH3 has a chiral centre. Which one of the following represents its R-configuration?
37
The order of decreasing reactivity towards an electrophilic reagent, for the following would be
(i) benzene
(ii) toluene
(iii) chlorobenzene
(iv) phenol
(i) benzene
(ii) toluene
(iii) chlorobenzene
(iv) phenol
38
For (i) I$$-$$, (ii) Cl$$-$$, (iii) Br$$-$$, the increasing order of nucleophilicity would be
39
Predict the product C obtained in the following reaction of 1-butyne.


40
Which of the compound with molecular formula C5H10 yields acetone on ozonolysis?
41
If there is no rotation of plane polarised light by a compound in a specific solvent, through to be chiral, it may mean than
Physics
1
A small coin is resting on the bottom of a beaker filled with liquid . A ray of light from the coin travels upto the surface of the liquid and moves along its surface. How fast is the light travelling in the liquid ?


2
The total power dissipated in watt in the circuit shown here is
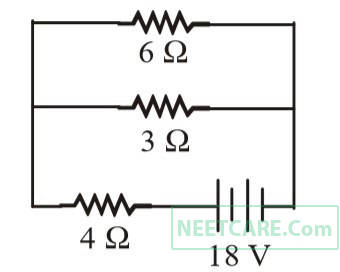

3
Three resistances, P, Q, R each of 2$$\Omega $$ and an unknown resistance S from the four arms of a Wheatstone bridge circuit. When a resistance of 6 $$\Omega $$ is connected in parallel to S the bridge gets balanced. What is the value of S?
4
Under the influence of a uniform magnetic field, a charged particle moves with constant speed v in a circle of radius R. The time period of rotation of the particle
5
The resistance of an ammeter is 13 $$\Omega $$ and its scale is graduated for a current upto 100 amps. After an additional shunt has been connected to this ammeter it becomes possible to measure currents upto 750 amperes by this meter. The value of shunt-resistance is
6
Nickel shows ferromagnetic property at room temperature. If the temperature is increased beyond Curie temperature, then it will show
7
A charged particle (charge q) is moving in a circle of radius R with uniform speed v. The associated magnetic moment $$\mu $$ is given by
8
A transformer is used to light a 100 W and 110 V lamp from a 220 V mains. If the main current is 0.5 amp, the efficiency of the transformer is approximately
9
What is the value of inductance L for which the current is maximum in a series LCR circuit with C = 10 $$\mu $$F and $$\omega $$ = 1000 s$$-$$1 ?
10
The primary and secondary coils of a transformer have 50 and 1500 turns respectively. If the magnetic flux $$\phi $$ linked with the primary coil is given by $$\phi $$ = $$\phi $$0 + 4t, where $$\phi $$ is webers, t is time in seconds and $$\phi $$0 is a constant, the output voltage across the secondary coil is
11
The electric and magnetic field of an electromagnetic wave are
12
Two condensers, one of capacity C and other of capacity C/2 are connected to a V-volt battery, as shown in the figure. The work done in charging fully both the condensers is


13
The frequency of a light wave in a material is 2 $$ \times $$ 1014 Hz and wavelength is 5000 $$\mathop A\limits^ \circ $$. The refractive index of material will be
14
Monochromatic light of frequency 6.0 $$ \times $$ 1014 Hz is produced by a laser. The power emitted is 2 $$ \times $$ 10$$-$$3 W. The number of photons emitted, on the average, by the source per second is
15
A beam of electron passes undeflected through mutually perpendicular electric and magnetic fields. If the electric field is switched off, and the same magnetic field is maintained, the electrons move
16
A 5 watt source emits monochromatic light of wavelength 5000 $$\mathop A\limits^ \circ $$. When placed 0.5 m away, it liberates photoelectrons from a photosensitive metallic surface. When the source is moved to a distance of 1.0 m, the number of photoelectrons liberated will be reduced by a factor of
17
In a mass spectrometer used for measuring the masses of ions, the ions are initially accelerated by an electric potential $$V$$ and then made to describe semicircular paths of radius R using a magnetic field B. If V and B are kept constant, the ratio $$\left( {{{ch\arg e\,\,on\,\,\,the\,\,ion\,\,} \over {mass\,\,of\,\,the\,\,ion}}} \right)$$ will be proportional to
18
A nucleus $${}_Z^AX$$ has mass represented by M(A, Z). If Mp and Mn denote the mass of proton and neutron respectively and B.E. the binding energy in MeV, then
19
The total energy of electron in the ground state of hydrogen atom is $$-$$ 13.6 eV. The kinetic energy of an electron in the first excited state is
20
Two satellites of earth, S1 and S2 are moving in the same orbit. The mass of S1 is four times the mass of S2. Which one of the following statements is true ?
21
A particle moving along x-axis has acceleration f, at time t, given by f = f0$$\left( {1 - {t \over T}} \right),$$ where f0 and T are constants.The particle at t = 0 has zero velocity. In the time interval between t = 0 and the instant when f = 0, the particle's velocity (vx) is
22
A car moves from X to Y with a uniform speed vu and returns to Y with a uniform speed vd. The average speed for this round trip is
23
The positions x of a particle with respect to time t along x-axis is given by x = 9t2 $$-$$ t3 where x is in metres and t in seconds. What will be the position of this particle when it achieves maximum speed along the + x direction ?
24
$$\overrightarrow A $$ and $$\overrightarrow B $$ are two vectors and $$\theta $$ is the angle between them, if $$\left| {\overrightarrow A \times \overrightarrow B } \right| = \sqrt 3 \left( {\overrightarrow A .\overrightarrow B } \right),$$ the value of $$\theta $$ is
25
A particle starting from the origin (0, 0) moves in a straight line in the (x, y) planes. Its coordinates at a later time are $$\left( {\sqrt 3 ,3} \right)$$. The path of the particle makes with the x-axes an angle of
26
A block B is pushed momentarily along a horizontaly surface with an initial velocity V. If $$\mu $$ is the coefficient of sliding friction between B and the surface, block B will come to rest after a time


27
A vertical spring with force constant k is fixed on a table. A ball of mass m at a height h above the free upper end of the spring falls vertically on the spring so that the spring is compressed by a distance d. The net work done in the process is
28
A particle of mass m moves in the XY plane with a velocity v along the straight line AB. If the angular momentum of the particle with respect to origin O is LA when it is at A and LB when it is at B, then


29
A uniform rod AB of length $$l$$ and mass m is free to rotate about point A. The rod is released from rest in the horizontal position. Given that the moment of inertia of the rod about A is ml2/3, the initial angular acceleration of the rod will be


30
A wheel has angular acceleration of 3.0 rad/sec2 and an initial angular speed of 2.00 rad/sec. In a time of 2 sec it has rotated through an angle (in radian) of
31
Which one of the following represents forward bias diode ?
32
Assuming the sun to have a spherical outer surface of radius r, radiating like a black body at temperature toC, the power received by a unit surface, (normal to the incident rays) at a distance R from the centre of the sun is
where $$\sigma $$ is the Stefan's constant.
where $$\sigma $$ is the Stefan's constant.
33
A black body is at 727oC. It emits energy at a rate which is proportional to
34
An engine has an efficiency of 1/6. When the temperature of sink is reduced by 62oC, its efficiency is doubled. Temperatures of the source is
35
A particle executes simple harmonic oscillation with an amplitude $$a$$. The period of oscillation is T. The minimum time taken by the particle to travel half of the amplitude from the equilibrium position is
36
A mass of 2.0 kg is put on a flat pan attached to a vertical spring fixed on the ground as shown in the figure. The mass of the spring and the pan is negligible.
When pressed slightly and released the mass executes a simple harmonic motion. The spring constant is 200 N/m. What should be the minimum amplitude of the motion so that the mass gets detached from the pan (take g = 10 m/s2).
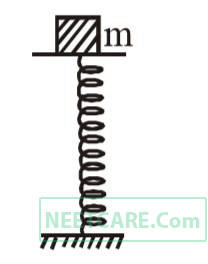
When pressed slightly and released the mass executes a simple harmonic motion. The spring constant is 200 N/m. What should be the minimum amplitude of the motion so that the mass gets detached from the pan (take g = 10 m/s2).

37
The particle executing simple harmonic motion has a kinetic energy K0cos2$$\omega $$t. The maximum values of the potential energy and the total energy are respectively
38
The phase difference between the instantaneous velocity and acceleration of a particle executing simple harmonic motion is
39
Charges +q and $$-$$q are placed at points A and B respectively which are a distance 2L apart, C is the midnight between A and B. The work done in moving a charge + Q along the semicircle CRD is


40
A hollow cylinder has a charge q coulomb within it. If $$f$$ is the electric flux in units of voltmeter associated with the curved surface B, the flux linked with the plane surface A in units of V-m will be


41
Three point charges +q, $$-$$ 2q and + q are placed at points (x = 0, y = a, z = 0), (x = 0, y = 0, z = 0) and (x = $$a$$, y = 0, z = 0) respectively. The magnitude and direction of the electric dipole moment vector of this charge assembly are
42
In the following circuit, the output Y for all possible inputs A and B is expressed by the truth table.


43
For a cubic crystal structure which one of the following relations indicating the cell characteristics is correct ?
44
In the energy band diagram of a material shown below, the open circles and filled circles denote holes and electrons respectively. The material is


45
Dimensions of resistance in an electrical circuit, in terms of dimension of mass M, of length L, of time T and of current I, would be