AIPMT 2008
Paper was held on
Sun, May 11, 2008 10:00 AM
Biology
1
Which type of white blood cells are connected with the release of histamine and the natural anticoagulant heparin?
2
Electrons from excited chlorophyll molecule of photosystem II are accepted first by
3
In leaves of C4 plants malic acid synthesis during CO2 fixation occurs in
4
The C4 plants are photosynthetically more efficient than C3 plants because
5
The energy-releasing metabolic process in which substrate is oxidised without an external electron acceptor is called
6
The chemiosmotic coupling hypothesis of oxidative phosphorylation proposes that adenosine triphosphate (ATP) is formed because
7
Importance of day length in flowering of plants was first shown in
8
Senescence as an active developmental cellular process in growth and functioning of a flowering plant, is indicated in
9
In humans, blood passes from the post caval to the diastolic right atrium of heart due to
10
The most active phagocytic white blood cells are
11
Cornea transplant in humans is almost never rejected. This is because
12
Which one of the following is the correct difference between rod cells and cone cells of our retina?
13
During the propagation of a nerve impulse, the action potential results from the movement of
14
Which one of the following pair of organs includes only the endocrine glands?
15
The blood calcium level is lowered by the deficiency of
16
Which one of the following is resistant to enzyme action?
17
What does the filiform apparatus do at the entrance into ovule?
18
Which one of the following pairs of plant structures has haploid number of chromosomes ?
19
Unisexuality of flowers prevents
20
In humans, at the end of the first meiotic
division, the male germ cells differentiate into
the:
21
In human adult females oxytocin:
22
Which one of the following pairs of items correctly belongs to the category of organs mentioned against it?
23
Bacterial leaf blight of rice is caused by a species
24
Thermococcus, Methanococcus and Methanobacterium exemplify
25
In the light of recent classification of living organisms into three domains of life (bacteria, archaea and eukarya), which one of the following statements in true about archaea?
26
Select one of the following pairs of important features distinguishing Gnetum from Cycas and Pinus and showing affinities with angiosperms.
27
In which one of the following male and female gametophytes do not have free living independent existance?
28
Which one of the following is heterosporous?
29
Which one of the following in birds, indicates their reptilian ancestry?
30
Which one of the following groups of three animals each is correctly matched with their one characteristic morphological feature?
31
Ascaris is characterized by
32
Which one of the following statements is
incorrect about menstruation?
33
Which one of the following is not a characteristic of Phylum Annelida?
34
Which one of the following phyla is correctly matched with its two general characteristics?
35
Replum is present in the ovary of flower of
36
The fleshy receptacle of syconus of fig encloses a number of
37
Endosperm is consumed by developing embryo in the sead of
38
The fruit is chambered, developed from inferior ovary and has seeds with succulent testa in
39
Dry indehiscent single-seeded fruit formed from bicarpellary syncarpous inferior ovary is
40
Carbohydrates are commonly found as starch in plants storage organs. Which of the following five properties of starch (1 $$-$$ 5) make it useful as a storage material?
(1) Easily translocated
(2) Chemically non-reactive
(3) Easily digested by animals
(4) Osmotically inactive
(5) Synthesized during photosynthesis
The useful properties are
(1) Easily translocated
(2) Chemically non-reactive
(3) Easily digested by animals
(4) Osmotically inactive
(5) Synthesized during photosynthesis
The useful properties are
41
Cellulose is the major component of cell walls of
42
A competitive inhibitor of succinic dehydrogenase is
43
Modern detergents contain enzyme preparations of
44
Cry I endotoxins obtained from Bacillus
thuringiensis are effective against:
45
Human insulin is being commercially produced
from a transgenic species of:
46
Consider the following four statements (1-4) about
certain desert animals such as kangaroo-rat.
(i) They have dark colour and high rate of reproduction and excrete solid urine
(ii) They do not drink water, breathe at a slow rate to conserve water and have their body covered with thick hairs
(iii) They feed on dry seeds and do not required drinking water
(iv) They excrete very concentrated urine and do not use water to regulate body temperature
Out of these four, which two are correct-
(i) They have dark colour and high rate of reproduction and excrete solid urine
(ii) They do not drink water, breathe at a slow rate to conserve water and have their body covered with thick hairs
(iii) They feed on dry seeds and do not required drinking water
(iv) They excrete very concentrated urine and do not use water to regulate body temperature
Out of these four, which two are correct-
47
Quercus species are the dominant component in:
48
About 70% of total global carbon is found in:
49
Consider the following statements concerning
food chains:
(i) Removal of 80% tigers from an area resulted in greatly increased growth of vegetation
(ii) Removal of most of the carnivores resulted in an increased population of deers
(iii) The length of food chains is generally limited to 3-4 trophic levels due to energy loss
(iv) The length of food chains may vary from 2 to 8 trophic levels
Which of two of the above statements are correct ?
(i) Removal of 80% tigers from an area resulted in greatly increased growth of vegetation
(ii) Removal of most of the carnivores resulted in an increased population of deers
(iii) The length of food chains is generally limited to 3-4 trophic levels due to energy loss
(iv) The length of food chains may vary from 2 to 8 trophic levels
Which of two of the above statements are correct ?
50
The slow rate of decomposition of fallen logs in
nature is due to their:
51
The table below gives the populations
(in thousands) of ten species (A-J) in four areas
(a-d) consisting of the number of habitats given
within brackets against each. Study the table
and answer the question which follows:


52
Which one of the following is not observed in
biodiversity hotspots ?
53
World Summit on Sustainable Development
(2002) was held in:
54
Main objective of production/use of herbicide
resistant GM crops is to:
55
The length of different internodes in a culm of
sugarcane is variable because of:
56
Vascular tissues in flowering plants develop
from:
57
Earthworms have no skeleton but during
burrowing the anterior end becomes turgid and
acts as a hydraulic skeleton. It is due to:
58
Which one of the following is the true
description about an animal concerned?
59
Vacuole in a plant cell:
60
The two sub-units of ribosome remain united at a
critical ion level of :
61
In germinating seeds fatty acids are degraded
exclusively in the:
62
Keeping in view the "fluid mosaic model" for
the structure of cell membrane, which one of the
following statements is correct with respect to
the movement of lipids and proteins from one
lipid monolayer to the other (described as flipflop movement)?
63
Given below is a diagrammatic cross section
of a single loop of human cochlea.
 Which one of the following options correctly
represents the names of three different parts?
Which one of the following options correctly
represents the names of three different parts?
 Which one of the following options correctly
represents the names of three different parts?
Which one of the following options correctly
represents the names of three different parts?64
A transgenic food crop which may help in
solving the problem of night blindness in
developing countries is
65
Which one of the following scientist's name is
correctly matched with the theory put fourth by
him?
66
Which extraembryonic membrane in humans
prevents desiccation of the embryo inside the
uterus?
67
Consider the statements given below regarding
contraception and answer as directed thereafter:
(i) Medical Termination of Pregnancy (MTP) during first trimester is generally safe
(ii) Generally chances of conception are nil until mother breast-feeds the infant upto two years
(iii) Intrauterine devices like copper-T are effective contraceptives
(iv) Contraception pills may be taken upto one week after coitus to prevent conception
Which two of the above statement are correct?
(i) Medical Termination of Pregnancy (MTP) during first trimester is generally safe
(ii) Generally chances of conception are nil until mother breast-feeds the infant upto two years
(iii) Intrauterine devices like copper-T are effective contraceptives
(iv) Contraception pills may be taken upto one week after coitus to prevent conception
Which two of the above statement are correct?
68
Given below are four methods (A-D) and their
modes of action (1-4) in achieving
contraception. Select their correct matching
from the four options that follow:
| Method | Mode of Action |
|---|---|
| (1) The pill | (a) Prevents sperms reaching cervix |
| (2) Condom | (b) Prevents implantation |
| (3) Vasectomy | (c) Prevents ovaulation |
| (4) Coppe | (d) Semen contains no sperms |
69
Which one of the following conditions in
humans is correctly matched with its
chromosomal abnormality/linkage?
70
In the DNA molecule:
71
Which one of the following pairs of nitrogenous
bases of nucleic acids, is wrongly matched with
the category mentioned against it?
72
Which one of the following pairs of codons is
correctly matched with their function or the
signal for the particular amino acid ?
73
Polysome is formed by
74
Darwin's finches are a good example of –
75
Thorn of Bougainvillea and tendril of Cucurbita
are examples of:
76
Which one of the following is incorrect about
the characteristics of protobionts (coacervates
and microspheres) as envisaged in the abiogenic
origin of life?
77
Match the disease in Column I with the
appropriate items (pathogen/ prevention/
treatment) in Column II.
| Column I | Column II |
|---|---|
| (A) Amoebiasis | (i) Treponema pallidium |
| (B) Diphtheria | (ii) Use only sterilized food |
| (C) Cholera | (iii) DPT Vaccine |
| (D) Syphilis | (iv) Use oval rehydration |
78
Which one of the following is the correct
statement regarding the particular psychotropic
drug specified ?
79
Haploids are more suitable for mutation studies
than the diploids. This is because:
80
Trichoderma harzianum has proved a useful
microorganism for:
81
Nitrogen fixation in root nodules of Alnus is
brought about by:
82
Which one of the following proved effective for
biological control of nematodal disese in plants?
83
Gel electrophoresis is used for:
84
The linking of antibiotic resistance gene with
the plasmid vector became possible with:
Chemistry
1
In which of the following coordination entities the magnitude of $$\Delta $$o (CFSE in octahedral field) will be maximum?
(At. No. Co = 27)
(At. No. Co = 27)
2
Which of the following complexes exhibits the highest paramagnetic behaviour?
where gly = glycine, en = ethylenediamine and bpy = bipyridyl moities.
(At. nos. Ti = 22, V = 23, Fe = 26, Co = 27)
where gly = glycine, en = ethylenediamine and bpy = bipyridyl moities.
(At. nos. Ti = 22, V = 23, Fe = 26, Co = 27)
3
How many moles of lead (II) chloride will be formed from a reaction between 6.5 g of PbO and 3.2 g HCl ?
4
In a SN2 substitution reaction of the type
R $$-$$ Br + Cl$$-$$ $$\buildrel {DMF} \over \longrightarrow $$ R$$-$$Cl + Br$$-$$
which one of the following has the highest relative rate?
R $$-$$ Br + Cl$$-$$ $$\buildrel {DMF} \over \longrightarrow $$ R$$-$$Cl + Br$$-$$
which one of the following has the highest relative rate?
5
Number of moles of MnO$$_4^{ - }$$ required to oxidize one mole of ferrous oxalate completely in acidic medium will be
6
A strong base can abstract an $$\alpha $$-hydrogen from
7
The relative reactivities of acyl compounds towards nucleophilic substitution are in the order of
8
Acetophenone when reacted with a base, C2H5ONa, yields a stable compound which has the structure
9
In a reaction of aniline a coloured product C was obtained.

The structure of C would be

The structure of C would be
10
In DNA, the complimentary bases are
11
Which of the following is an amine hormone?
12
How many stereoisomers does this molecule have?
CH3CH$$=$$CHCH2CHBrCH3
CH3CH$$=$$CHCH2CHBrCH3
13
On the basis of the following Eo values, the strongest oxidizing agent is
[Fe(CN)6]4$$-$$ $$ \to $$ [Fe(CN)6]3$$-$$ + e$$-$$; Eo = $$-$$0.35 V
Fe2+ $$ \to $$ Fe3+ + e$$-$$; Eo = $$-$$0.77 V
[Fe(CN)6]4$$-$$ $$ \to $$ [Fe(CN)6]3$$-$$ + e$$-$$; Eo = $$-$$0.35 V
Fe2+ $$ \to $$ Fe3+ + e$$-$$; Eo = $$-$$0.77 V
14
Kohlrausch's law states that at
15
Standard free energies of formation (in kJ/mol) at 298 K are $$-$$237.2, $$-$$ 394.4 and $$-$$8.2 for H2O(l), CO2(g) and pentane (g) respectively. The value of Eocell for the pentane-oxygen fuel cell is
16
The rate constants k1 and k2 for two different reactions are 1016 $$ \cdot $$ e$$-$$2000/T and 1015 $$ \cdot $$ e$$-$$1000/T, respectively.
The temperature at which k1 = k2 is
The temperature at which k1 = k2 is
17
The bromination of acetone that occurs in acid solution is represented by this equation.
CH3COCH3(aq) + Br2(aq) $$ \to $$
CH3COCH2Br(aq) + H+(aq) + Br$$-$$(aq)
These kinetic data were obtained for given reaction concentrations.
Based on these data, the rate equation is
CH3COCH3(aq) + Br2(aq) $$ \to $$
CH3COCH2Br(aq) + H+(aq) + Br$$-$$(aq)
These kinetic data were obtained for given reaction concentrations.
| Initial concentrations, M | ||||
|---|---|---|---|---|
| [CH3COCH3 | [Br2] | [H+] | ||
| 0.30 | 0.05 | 0.05 | ||
| 0.30 | 0.10 | 0.05 | ||
| 0.30 | 0.10 | 0.10 | ||
| 0.40 | 0.05 | 0.20 | ||
| Initial rate, disappearance of Br2, Ms$$-$$1 | ||||||
|---|---|---|---|---|---|---|
| 5.7$$ \times $$10$$-$$5 | ||||||
| 5.7$$ \times $$10$$-$$5 | ||||||
| 1.2$$ \times $$10$$-$$4 | ||||||
| 3.1$$ \times $$10$$-$$4 | ||||||
Based on these data, the rate equation is
18
The angular shape of ozone molecule (O3) consists of
19
The correct order of decreasing second ionisation enthalpy of Ti(22), V(23), Cr(24) and Mn(25) is
20
For the gas phase reaction,
PCl5(g) $$\rightleftharpoons$$ PCl3(g) + Cl2(g)
which of the following conditions are correct ?
PCl5(g) $$\rightleftharpoons$$ PCl3(g) + Cl2(g)
which of the following conditions are correct ?
21
An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave C, 38.71% and H, 9.67%. The empirical formula of the compound would be
22
What volume of oxygen gas (O2) measuread at 0oC and 1 atm, is needed to burn completely 1 L of propane gas (C3H8) measured under the same conditions ?
23
The measurement of the electron position is associated with an uncertainty in momentum, which is equal to 1 $$ \times $$ 10$$-$$18 g cm s$$-$$1. The uncertainty in electron velocity is (mass of an electron is 9 $$ \times $$ 10$$-$$28 g)
24
If uncertainty in position and momentum are equal, then uncertainty in velocity is
25
Which one of the following arrangements does not give the correct picture of the trends indicated against it ?
26
Four diatomic species are listed below. Identify the correct order in which the bond order is increasing in them
27
The correct order of increasing bond angles in the following triatomic species is
28
Which of the following are not state functions ?
(I) q + w (II) q
(III) w (IV) H $$-$$ TS
(I) q + w (II) q
(III) w (IV) H $$-$$ TS
29
Bond dissociation enthalpy of H2, Cl2 and HCl are 434, 242 and 431 kJ mol$$-$$1 respectively. Enthalpy of formation of HCl is
30
The dissociation equilibrium of a gass AB2 can be represented as :
2AB2(g) $$\rightleftharpoons$$ 2AB(g) + B2(g)
The degree of dissociation is x and is small compared to 1. The expression relating the degree of dissociation (x) with equilibrium constant Kp and total pressure P is
2AB2(g) $$\rightleftharpoons$$ 2AB(g) + B2(g)
The degree of dissociation is x and is small compared to 1. The expression relating the degree of dissociation (x) with equilibrium constant Kp and total pressure P is
31
If the concentration of OH$$-$$ ions in the reaction
Fe(OH)3(s) $$\rightleftharpoons$$ Fe3+(aq) + 3OH$$-$$(aq)
is decreased by 1/4 times, then equilibrium concentration of Fe3+ will increase by
Fe(OH)3(s) $$\rightleftharpoons$$ Fe3+(aq) + 3OH$$-$$(aq)
is decreased by 1/4 times, then equilibrium concentration of Fe3+ will increase by
32
The value of equilibrium constant of the reaction
HI(g) $$\rightleftharpoons$$ $${1 \over 2}$$H2(g) + $${1 \over 2}$$I2(g)
is 8.0. The The equilibrium constant of the reaction
H2(g) + I2(g) $$\rightleftharpoons$$ 2HI(g) will be
HI(g) $$\rightleftharpoons$$ $${1 \over 2}$$H2(g) + $${1 \over 2}$$I2(g)
is 8.0. The The equilibrium constant of the reaction
H2(g) + I2(g) $$\rightleftharpoons$$ 2HI(g) will be
33
The values of for the reactions,
X $$\rightleftharpoons$$ Y + Z . . . .(i)
A $$\rightleftharpoons$$ 2B . . . .(ii)
are in the ratio 9 : 1. If degree of dissociation of X and A be equal, then total pressure at equilibrium (i) and (ii) are in the ratio
X $$\rightleftharpoons$$ Y + Z . . . .(i)
A $$\rightleftharpoons$$ 2B . . . .(ii)
are in the ratio 9 : 1. If degree of dissociation of X and A be equal, then total pressure at equilibrium (i) and (ii) are in the ratio
34
Equal volumes of three acid solutions of pH 3, 4 and 5 are mixed in a vessel. What will be the H+ ion concentration in the mixture?
35
Base strength of

is in the order of

is in the order of
36
Which one of the following is most reactive towards electrophilic attack?
37
The stability of carbanions in the following.

is in the order of

is in the order of
38

A (predominantly) is
39
In the hydrocarbon,

The state of hybridization of carbons 1, 3 and 5 are in the following sequence

The state of hybridization of carbons 1, 3 and 5 are in the following sequence
Physics
1
Two thin lenses of focal lengths f1 and f2 are in contact and coaxial. The power of the combination is
2
An electric kettle takes 4 A current at 220 V. How much time will it take to boil 1 kg of water from temperature 20oC ? The temperature of boiling water is 100oC
3
A current of 3 amp. flows through the 2 $$\Omega $$ resistor shown in the circuit. The power dissipated in the 5 $$\Omega $$ resistor is
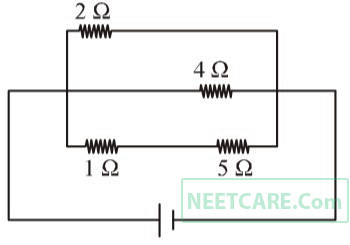

4
A cell can be balanced against 110 cm and 100 cm of potentiometer wire, respectively with and without being short circuited through a resistance of 10 $$\Omega $$. Its internal resistance is
5
A particle of mass m, charge Q and kinetic energy T enters a transverse uniform magnetic field of induction $$\overrightarrow B $$. After 3 seconds the kinetic energy of the particle will be
6
A closed loop PQRS carrying a current is placed in a uniform magnetic field. If the magnetic forces on segments PS, SR and RQ are F1, F2 and F3 respectively and are in the plane of the paper and along the directions shown, the force on the segment QP is


7
A galvanometer of resistance 50 $$\Omega $$ is connected to a battery of 3 V along with a resistance of 2950 $$\Omega $$ in series. A full scale deflection of 30 divisions is obtained in the galvanometer. In order to reduce this deflection to 20 divisions, the resistance in series should be
8
Curie temperature above which
9
A long solenoid has 500 turns. When a current of 2 ampere is passed through it, the resulting magnetic flux linked with each turn of the solenoid is 4 $$ \times $$ 10$$-$$3 Wb. The self-inductance of the solenoid is
10
In an a.c. circuit the e.m.f. ($$\varepsilon $$) and the current
(i) at any instant are given respectively by
$$\varepsilon $$ = E0sin$$\omega $$t, $$i$$ = $$I$$0sin($$\omega $$t $$-$$ $$\phi $$)
The average power in the circuit over one cycle of a.c. is
(i) at any instant are given respectively by
$$\varepsilon $$ = E0sin$$\omega $$t, $$i$$ = $$I$$0sin($$\omega $$t $$-$$ $$\phi $$)
The average power in the circuit over one cycle of a.c. is
11
A circular disc of radius 0.2 meter is placed in a uniform magnetic field of induction $${1 \over \pi }\left( {{{Wb} \over {{m^2}}}} \right)$$ in such a way that its axis makes an angle of 60o with $$\overrightarrow B .$$ The magnetic flux linked with the disc is
12
The velocity of electromagnetic radiation in a medium of permittivity $$\varepsilon $$0 and permeability $$\mu $$0 is given by
13
A wire of a certain material is stretched slowly by ten percent. Its new resistance and specific resistance become respectively
14
A boy is trying to start a fire by focusing sunlight on a piece of paper using an equiconvex lens of focal length 10 cm. The diameter of the sun is 1.39 $$ \times $$ 109 m and its mean distance from the earth is 1.5 $$ \times $$ 1011 m. What is the diameter of the sun's image on the paper ?
15
A particle of mass 1 mg has the same wavelength as an electron moving with a velocity of 3 $$ \times $$ 106 m s$$-$$1. The velocity of the particle is
16
The work function of a surface of a photosensitive material is 6.2 eV. The wavelength of the incident radiation for which the stopping potential is 5 V lies in the
17
In the phenomenon of electric discharge through gases at low pressure, the coloured glow in the tube appears as a result of
18
If M(A; Z), Mp and Mn denote the masses of the nucleus $${}_Z^AX,$$ proton and neutron respectively in units of u (1 u = 931.5 MeV/c2) and BE represents its bonding energy in MeV, then
19
The ground state energy of hydrogen atom is $$-$$ 13.6 eV. When its electron is in the first excited state, its excitation energy is
20
A p-n photodiode is made of a material with a band gap of 2.0 eV. The minimum frequency of the radiation that can be absorbed by the material is nearly
21
If the lattice parameter for a crystalline structure is 3.6 $$\mathop A\limits^ \circ $$, then the atomic radius in fcc crystal is
22
The ratio of the radii of gyration of a circular disc to that of a circular ring, each of same mass and radius, around their respective axes is
23
Which two of the following five physical parameters have the same dimensions ?
1. Energy density
2. Refractive index
3. Dielectric constant
4. Young's modulus
5. Magnetic field
1. Energy density
2. Refractive index
3. Dielectric constant
4. Young's modulus
5. Magnetic field
24
A particle moves in a straight line with a constant acceleration. It changes its velocity from 10 ms$$-$$1 to 20 ms$$-$$1 while passing through a distance 135 m in t second. The value of t is
25
The distance travelled by a particle starting from rest and moving with an acceleration $${4 \over 3}$$m s$$-$$2, in the third second is
26
A particle of mass m is projected with velocity v making an angle of 45o with the horizontal. When the particle lands on the level ground the magnitude of the change in its momentum will be :
27
A particle shows distance - time curve as given in this figure. The maximum instaneous velocity of the particle is around the point


28
A roller coaster is designed such that riders experience ''weightlessness'' as they go round the top of a hill whose radius of curvature is 20 m. The speed of the car at the top of the hill is between
29
Sand is being dropped on a conveyer belt at the rate of M kg/s. The force necessary to keep the belt moving with a constant velocity of $$\upsilon $$ m/s will be :
30

Three forces acting on a body are shown in the figure. To have the resultant force only along the y-direction, the magnitude of the minimum additional force needed is
31
Water falls from a height of 60 m at the rate of 15 kg/s to operate a turbine. The losses due to frictional forces are 10% of energy. How much power is generated by the turbine ? (g = 10 m/s2)
32
A shell of mass 200 gm is ejected from a gun of mass 4 kg by an explosion that generates 1.05 kJ of energy. The initial velocity of the shell is :
33
A thin rod of length L and mass M is bent at its midpoint into two halves so that the angle between them is 90o. The moment of inertia of the bent rod about an axis passing through the bending point and perpendicular to the plane defined by the two halves of the rod is
34
On a new scale of temperature (which is linear) and called the W scale, the freezing and boiling points of water are 39oW and 239oW respectively. What will be the temperature on the new scale, corresponding to a temperature of 39oC on the Celsius scale ?
35
At 10oC the value of the density of a fixed mass of an ideal gas divided by it pressure is x. At 110oC this ratio is
36
If Q, E and W denote respectively the heat added, change in internal energy and the work done in a closed cyclic process, then
37
Two simple harmonic motions of angular frequency 100 and 1000 rad s$$-$$1 have the same displacement amplitude. The ratio of their maximum acceleration is
38
A point performs simple harmonic oscillation of period T and the equation of motion is given by x = a sin($$\omega $$t + $$\pi $$/6). After the elapse of what fraction of the time period the velocity of the point will be equal to half of its maximum velocity?
39
Two periodic waves of intensities $$I$$1 and $$I$$2 pass through a region at the same time in the same direction. The sum of the maximum and minimum intensities is
40
The wave described by y = 0.25 sin(10$$\pi $$x $$-$$ 2$$\pi $$t), where x and y are in metres and t in seconds, is a wave travelling along the
41
A parallel plate capacitor has a uniform electric field E in the space between the plates. If the distance between the plates is d and area of each plate is A, the energy stored in the capacitor is
42
A thin conducting ring of radius R is given a charge +Q. The electric field at the centre O of the ring due to the charge on the part AKB of the ring is E. The electric field at the centre due to the charge on the part ACDB of the ring is


43
The electric potential at a point in free space due to charge Q coulomb is Q $$ \times $$ 1011 volts. The electric field at that point is
44
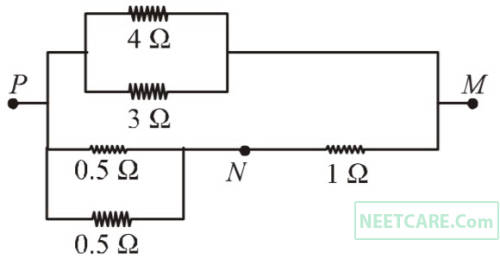
In the circuit shown, the current through the 4 $$\Omega $$ resistor is 1 amp when the points P and M are connected to a d.c. voltage source. The potential difference betwen the points M and N is
45
If the error in the measurement of radius of a sphere is 2%, then the error in the determination of volume of the sphere will be