AIPMT 2009
Paper was held on
Sun, May 10, 2009 10:00 AM
Biology
1
What will happen if the stretch receptors of the urinary bladder wall are totally removed?
2
One of the synthetic auxin is
3
Which one of the following acids is a derivative of carotenoids?
4
What is vital capacity of our lungs?
5
The haemoglobin of a human foetus
6
There is no DNA in
7
In a standard ECG which one of the following alphabets is the correct representation of the respective activity of the human heart?
8
Compared to blood our lymph has
9
Globulins contained in human blood plasma are primarily involved in
10
Aerobic respiratory pathway is appropriately termed
11
Uric acid is the chief nitrogenous component of the excretory products of
12
Elbow joint is an example of
13
Which one of the following is the correct matching of three items and their grouping category?
14
Which part of human brain is concerned with the regulation of body temperature?
15
Alzheimer's disease in humans is associated with the deficiency of
16
A health disorder that results from the deficiency of thyroxine in adults and characterised by (i) a low metabolic rate, (ii) increase in body weight and (iii) tendency to retain water in tissues is
17
Vegetative propagation in mint occurs by
18
Seminal plasma in humans is rich in :
19
Which one of the following is the correct matching
of the events occurring during menstrual cycle ?
20
Given below is a diagrammatic sketch for a
portion of human male reproductive system.
Select the correct set of the names of the parts
labelled A, B, C, D.


21
Foetal ejection reflex in human female is induced by
22
If a live earthworm is pricked with a needle on its outer surface without damaging its gut, the fluid that comes out is
23
Phylogenetic system of classification is based on
24
Which one is the wrong pairing for the disease and its causal organism?
25
Oxygenic photosynthesis occurs in
26
Which of the following is a symbiotic nitrogen fixer?
27
T.O. Diener discovered a
28
Which one of the following is a vascular cryptogam?
29
Which one of the following is considered important in the development of seed habit?
30
Mannitol is the stored food in
31
Which one of the following has haplontic life cycle?
32
Which one of the following plants is monoecious?
33
Which one of the following groups of animals is bilaterally symmetrical and triploblastic?
34
Which one of the following pairs of animals comprises 'jawless fishes'?
35
The correct sequence of spermatogenetic stages
leading to the formation of sperms in a mature
human testis is :
36
An examples of a seed with endosperm, perisperm, and caruncle is
37
Cotyledons and testa respectively are edible parts in
38
An example of axile placentation is
39
A fruit developed from hypanthodium inflorescence is called
40
The floral formula

is that of

is that of
41
Synapsis occurs between
42
Given below is a schematic break-up of the phases/stages of cell cycle. Which one of the following is the correct indication of the stage/phase in the cell cycle?


43
Cyclic photophosphorylation results in the formation of
44
Stroma in the chloroplasts of higher plant contains
45
Palisade parenchyma is absent in leaves of -
46
A country with a high rate of population growth
took measures to reduce it. The figure below
shows age-sex pyramids of populations A and B
twenty years apart. Select the correct
interpretation about them : –


47
Reduction in vascular tissue, mechanical tissue
and cuticle is characteristic of :
48
The correct sequence of plants in hydrosere is :
49
Which one of the following types organisms
occupy more than one trophic level in a pond
ecosystem ?
50
Which one of the following has maximum
genetic diversity in India ?
51
Tiger is not a resident in which one of the
following National Park ?
52
What is antisense technology?
53
In barley stem vascular bundles are -
54
The annular and spirally thickened conducting
elements generally develop in the protoxylem
when the root or stem is -
55
Anatomically fairly old dicotyledonous root is
distinguished from the dicotyledonous stem by :
56
Which one of the following correctly described
the location of some body parts in the earthworm
Pheretima ?
57
The kind of tissue that forms the supportive
structure in our pinna (external ears) is also
found in -
58
Which one of the following is correct pairing of
a body part and like kind of muscle tissue that
moves it ?
59
The cell junctions called tight, adhering and gap
junctions are found in :
60
The epithelial tissue present on the inner surface
of bronchioles and fallopian tubes is :
61
Middle lamella is composed mainly of :
62
Plasmodesmata are :
63
Cytoskeleton is made up of :
64
Peripatus is a connecting link between :
65
Which one of the following is the most likely
root cause why menstruation is not taking place
in regularly cycling human female ?
66
A change in the amount of yolk and its
distribution in the egg will effect :
67
Select the incorrect statement from the
following :
68
Sickle-cell anemia is :
69
The genetic defect-adenosine deaminase (ADA)
deficiency may be cured permanently by
70
The most popularly known blood grouping is
the ABO grouping. It is named ABO and not
ABC, because "O" in it refers to having :
71
Study the pedigree chart given below :
 What does it show ?
What does it show ?
 What does it show ?
What does it show ? 72
Semi-conservative replication of DNA was first
demonstrated in :
73
Whose experiments cracked the DNA and
discovered unequivocally that a genetic code is a
"triplet" ?
74
Removal of introns and joining the exons in a
defined order in a transcription unit is called :
75
What is not true for genetic code?
76
In the case of peppered moth (Biston betularia)
the black-coloured from became dominant over
the light-coloured form in England during
industrial revolution. This is an example of -
77
Which of following is a pair of viral diseases ?
78
Which one of the following statement is
correct ?
79
Polyethylene glycol method is used for :
80
Somaclones are obtained by : -
81
Which of the following is not used as a
biopesticide ?
82
Which one of the following pairs is wrongly
matched ?
83
Which one of the following is commonly used in
transfer of foreign DNA into crop plants ?
84
What is true about Bt toxin ?
85
Transgenic plants are the ones -
86
The bacterium Bacillus thuringiensis is widely
used in contemporary biology as -
Chemistry
1
Which of the following complex ions is expected to absorb visible light?
(At. nos. Zn = 30, Sc = 21, Ti = 22, Cr = 24)
(At. nos. Zn = 30, Sc = 21, Ti = 22, Cr = 24)
2
10 g of hydrogen and 64 g of oxygen were filled in a steel vessel and exploded. Amount of water produced in this reaction will be
3
Al2O3 is reduced by electrolysis at low potentials and high currents. If 4.0 $$ \times $$ 104 amperes of current is passed through molten Al2O3 for 6 hours, what mass of aluminium is product? (Assume 100% current efficiency, at mass of Al = 27 g mol$$-$$1).
4
Trichloroacetaldehyde, CCl3CHO reacts with chlorobenzene in presence of sulphuric acid and produces
5
Propionic acid with Br2/P yields a dibromo product. Its structure would be
6
Nitrobenzene can be prepared from benzene by using a mixture of conc. HNO3 and conc. H2SO4. In the mixture, nitric acid acts as a/an
7
Predict the product.


8
The segment of DNA which acts as the instrumental manual for the synthesis of the protein is
9
Which of the following hormones contains iodine?
10
A 0.0020 m aqueous solution of an ionic compound [Co(NH3)5(NO2)]Cl freezes at $$-$$ 0.00732oC. Number of moles of ions which 1 mol of ionic compound produces on being dissolved in water will be (Kf = $$-$$1.86oC/m)
11
Given :
(i) Cu2+ + 2e$$-$$ $$ \to $$ Cu, Eo = 0.337 V
(ii) Cu2+ + e$$-$$ $$ \to $$ Cu+, Eo = 0.153 V
Electrode potential, Eo for the reaction,
Cu+ + e$$-$$ $$ \to $$ Cu, will be
(i) Cu2+ + 2e$$-$$ $$ \to $$ Cu, Eo = 0.337 V
(ii) Cu2+ + e$$-$$ $$ \to $$ Cu+, Eo = 0.153 V
Electrode potential, Eo for the reaction,
Cu+ + e$$-$$ $$ \to $$ Cu, will be
12
The equivalent conductance of M/32 solution of a weak monobasic acid is 8.0 mho cm2 and at infinite dilution is 400 mho cm2. The dissociation constant of this acid is
13
For the reaction, N2 + 3H2 $$ \to $$ 2NH3, if
$${{d\left[ {N{H_3}} \right]} \over {dt}}$$ = 2 $$ \times $$ 10$$-$$4 mol L$$-$$1 s$$-$$1,
the value of $${{ - d\left[ {{H_2}} \right]} \over {dt}}$$ would be
$${{d\left[ {N{H_3}} \right]} \over {dt}}$$ = 2 $$ \times $$ 10$$-$$4 mol L$$-$$1 s$$-$$1,
the value of $${{ - d\left[ {{H_2}} \right]} \over {dt}}$$ would be
14
In the reaction,
BrO$$_{3(aq)}^ - $$ + 5Br$$_{(aq)}^ - $$ + 6H+ $$ \to $$ 3Br2(l) + 3H2O(l).
The rate of appearance of bromine (Br2) is related to rate of disappearance of bromide ions as
BrO$$_{3(aq)}^ - $$ + 5Br$$_{(aq)}^ - $$ + 6H+ $$ \to $$ 3Br2(l) + 3H2O(l).
The rate of appearance of bromine (Br2) is related to rate of disappearance of bromide ions as
15
For the reaction A + B $$ \to $$ products, it is observed that
(i) on doubling the initial concentration of A only, the rate of reaction is also doubled and
(ii) on doubling the initial concentration of both A and B, there is a change by a factor of 8 in the rate of the reaction.
The rate of this reaction is given by
(i) on doubling the initial concentration of A only, the rate of reaction is also doubled and
(ii) on doubling the initial concentration of both A and B, there is a change by a factor of 8 in the rate of the reaction.
The rate of this reaction is given by
16
Half-life period of a first order reaction is 1386 seconds. The specific rate constant of the reaction is
17
The stability of +1 oxidation state among Al, Ga, In and Tl increases in the sequence
18
The straight chain polymer is formed by
19
Among the following which is the strongest oxidising agent?
20
Which one of the elements with the following outer orbital configurations may exhibit the largest number of oxidation states?
21
Which of the following does not show optical isomerism?
(en = ethylenediamine)
(en = ethylenediamine)
22
Out of TiF62$$-$$, CoF63$$-$$, Cu2Cl2 and NiCl42$$-$$ (Z of Ti = 22, Co = 27, Cu = 29, Ni = 28) the colourless species are
23
What is the [OH$$-$$] in the final solution prepared by mixing 20.0 mL of 0.050 M HCl with 30.0 mL of 0.10 M Ba(OH)2?
24
Maximum number of electrons in a subshell of an atom is determined by the following
25
Which of the following is not permissible arrangement of electrons in an atom ?
26
Amongst the elements with following electronic configurations, which one of them may have the highest ionisation energy ?
27
What is the dominant intermolecular force or bond that must be overcome in converting liquid CH3OH to a gas?
28
In which of the following molecules/ions BF3, NO$$_2^ - $$, NH$$_2^ - $$ and H2O, the central atom is sp2 hybridised?
29
According to MO theory which of the lists ranks the nitrogen species in terms of increasing bond order ?
30
The values of $$\Delta $$H and $$\Delta $$S for the reaction,
C(graphite) + CO2(g) $$ \to $$ 2CO(g)
are 170 kJ and 170 J K$$-$$1, respectively. This reaction will be spontaneous at
C(graphite) + CO2(g) $$ \to $$ 2CO(g)
are 170 kJ and 170 J K$$-$$1, respectively. This reaction will be spontaneous at
31
From the following bond energies :
H $$-$$ H bond energy : 431.37 kJ mol$$-$$1
C $$=$$ C bond energy : 606.10 kJ mol$$-$$1
C $$-$$ C bond energy : 336.49 kJ mol$$-$$1
C $$-$$ H bond energy : 410.50 kJ mol$$-$$1
Enthalpy for the reaction,

will be
H $$-$$ H bond energy : 431.37 kJ mol$$-$$1
C $$=$$ C bond energy : 606.10 kJ mol$$-$$1
C $$-$$ C bond energy : 336.49 kJ mol$$-$$1
C $$-$$ H bond energy : 410.50 kJ mol$$-$$1
Enthalpy for the reaction,

will be
32
The dissociation constants for acetic acid and HCN at 25oC are 1.5 $$ \times $$ 10$$-$$5 and 4.5 $$ \times $$ 10$$-$$10 respectively. The equilibrium constant for the equilibrium
CN$$-$$ + CH3COOH $$\rightleftharpoons$$ HCN + CH3COO$$-$$ would be
CN$$-$$ + CH3COOH $$\rightleftharpoons$$ HCN + CH3COO$$-$$ would be
33
The ionization constant of ammonium hydroxide is 1.77 $$ \times $$ 10$$-$$5 at 298 K. Hydrolysis constant of ammonium chloride is
34
Which of the following molecules acts as a Lewis acid?
35
The IUPAC name of the compound having the formula
CH$$ \equiv $$C$$-$$CH$$=$$CH2 is
CH$$ \equiv $$C$$-$$CH$$=$$CH2 is
36
Benzene reacts with CH3Cl in the presence of anhydrons AlCl3 to form
37
Which of the following compounds will exhibit cis-trans (geometrical) isomerism?
38
The state of hybridisation of C2, C3, C5 and C6 of the hydrocarbon,

is in the following sequence

is in the following sequence
39
Which of the following reactions is an example of nucleophilic substitution reaction?
40
Consider the following reaction :

the product Z is

the product Z is
41
HOCH2CH2OH on heating with periodic acid gives
42
Consider the following reaction :

the product Z is

the product Z is
43
Oxidation numbers of P in PO$$_4^{3 - }$$, of S in SO$$_4^{2 - }$$ and that of Cr in Cr2O$$_7^{2 - }$$ are respectively
Physics
1
The electric field part of an electromagnetic wave in a medium is represented by Ex = 0;
$${E_y} = 2.5{N \over C}\cos \left[ {\left( {2\pi \times {{10}^6}{{rad} \over m}} \right)t - \left( {\pi \times {{10}^{ - 2}}{{rad} \over s}} \right)x} \right];$$
Ez = 0.
$${E_y} = 2.5{N \over C}\cos \left[ {\left( {2\pi \times {{10}^6}{{rad} \over m}} \right)t - \left( {\pi \times {{10}^{ - 2}}{{rad} \over s}} \right)x} \right];$$
Ez = 0.
2
See the electrical circuit shown in this figure. Which of the following equations is a correct equation for it ?
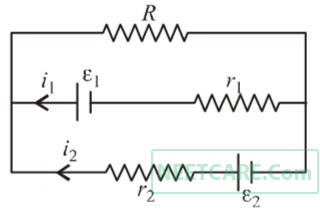

3
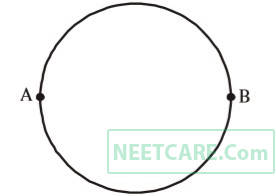
A wire of resistance 12 ohms per meter is bent to form a complete circle of radius 10 cm. The resistance between its two diametrically opposite points, A and B as shown in the figure is

4
The mean free path of electrons in a metal is 4 $$ \times $$ 10$$-$$8 m. The electric field which can give on an average 2 eV energy to an electron in the metal will be in units V/m
5
A student measures the terminal potential difference (V) of a cell (of emf $$\varepsilon $$ and internal resistance r) as a function of the current (I) flowing through it. The slope, and intercept, of the graph between V and I, then respectively, equal
6
A galvanometer havings a coil resistance of 60 $$\Omega $$ shows full scale deflection when a current of 1.0 amp passes through it. It can be converted into an ammeter to read currents upto 5.0 amp by
7
The magnetic force acting on a charged particle of charge $$-$$2 $$\mu $$C in a magnetic frield of 2 T acting in y direction, when the particle velocity is $$\left( {2\widehat i + 3\widehat j} \right) \times {10^6}\,m{s^{ - 1}}$$
8
Under the influence of a uniform magnetic field, a charged particle moves with constant speed v in a circle of radius R. The time period of rotation of the particle
9
If a diamagnetic substance is brought near the north or the south pole of a bar magnet, it is
10
A bar magnet having a magnetic moment of 2 $$ \times $$ 104 J T$$-$$1 is free to rotate in a horizontal plane. A horizontal magnetic field B = 6 $$ \times $$ 10$$-$$4 T exists in the space. The work done in taking the magnet slowly from a direction parallel to the field to a direction 60o from the field is
11
A rectangular, a square, a circular and an elliptical loop, all in the (x-y) plane, are moving out of a uniform magnetic field with a constant velocity. $$\overrightarrow V = v\widehat i$$. The magnetic field is directed along the negative z axis direction. The induced emf, during the passes of these loops, out of the field region, will not remain constant for
12
Power dissipated in an LCR series circuit connected to an A.C. source of emf $$\varepsilon $$ is
13
A conducting circular loop is placed in a uniform magnetic field 0.04 T with its plane perpendicular to the magnetic field. The radius of the loop starts shrinking at 2 mm/s. The induced emf in the loop when the radius is 2 cm is
14
The electric potential at a point (x, y, z) is given by V = $$-$$x2y $$-$$ xz3 + 4
The electric field at that point is
The electric field at that point is
15
The number of photo electrons emitted for light of a frequency $$\upsilon $$ (higher than the threshold frequency $${\upsilon _0}$$) is proportional to
16
Monochromatic light of wavelength 667 nm is produced by a helium neon laser. The power emitted is 9 mW. The number of photons arriving per sec. on the average at a target irradiated by this beam is
17
The figure shows a plot of photo current versus anode potential for a photo sensitive surface for three different radiations. Which one of the following is a correct statement?


18
In a Rutherford scattering experiment when a projectile of charge z1 and mass M1 approaches a target nucleus of charge z2 and mass M2, the distance of closest approach is r0. The energy of the projectile is
19
The ionization energy of the electron in the hydrogen atom in its ground state is 13.6 eV. The atoms are excited to higher energy levels to emit radiations of 6 wavelengths, Maximum wavelength of emitted radiation corresponds to the transition between
20
The symbolic representation of four logic gates are given below

The logic symbols for OR, NOT and NAND gates are respectively

The logic symbols for OR, NOT and NAND gates are respectively
21
A p-n photodiode is fabricated from a semiconductor with a band gap of 2.5 eV. It can detect a signal of wavelength
22
Sodium has body centred packing. Distance between two nearest atoms is 3.7 $$\mathop A\limits^ \circ $$. The lattice parameter is
23
The figure shows elliptical orbit of a planet m about the sun S. The shaded area SCD is twice the shaded area SAB. If t1 is the time for the planet to move from C to D and t2 is the time to move from A to B then


24
A bus is moving with a speed of 10 ms$$-$$1 on a straight road. A scooterist wishes to overtake the bus in 100 s. If the bus is at a distance of 1 km from the scooterist, with what speed should the scooterist chase the bus ?
25
A particle starts its motion from rest under the action of a constant force. If the distance covered in first 10 seconds is S1 and that covered in the first 20 seconds is S2, then
26
The mass of a lift is 2000 kg. When the tension in the supporting cable is 28000 N, then its acceleration is :
27
A body, under the action of a force $$\overrightarrow F = 6\widehat i - 8\widehat j + 10\widehat k,$$ acquires an accelerations of 1 m/s2. The mass of this body must be
28
A body of mass 1 kg is thrown upwards with a velocity 20 m/s. It momentarily comes to rest after attaining a height of 18 m. How much energy is lost due to air friction? (g = 10 m/s2)
29
An explosion blows a rock into three parts. Two parts go off at right angles to each other. These two are, 1 kg first part moving with a velocity of 12 m s$$-$$1 and 2 kg second part moving with a velocity 8 m s$$-$$1. If the third part flies off with a velocity of 4 m s$$-$$1, its mass would be :
30
A block of mass M is attached to the lower end of a vertical spring. The spring is hung from a ceiling and has force constant value k. The mass is released from rest with the spring initially unstretched. The maximum extension produced in the length of the spring will be
31
An engine pumps water continuously through a hose. Water leaves the hose with a velocity v and m is the mass per unit length of the water jet. What is the rate at which kinetic energy is imparted to water?
32
A thin circular ring of mass M and radius R is rotating in a horizontal plane about an axis vertical to its plane with a constant angular velocity $$\omega $$. If two objects each of mass m be attached gently to the opposite ends of a diameter of the ring, the ring will then rotate with an angular velocity
33
Four identical thin rods each of mass M and length $$l$$, form a square frame. Moment of inertia of this frame about an axis through the centre of the square and perpendicular to its plane is
34
If $$\overrightarrow F $$ is the force acting on a particle having position vector $$\overrightarrow r $$ and $$\overrightarrow \tau $$ be the torque of this force about the origin, then
35
Two bodies of mass 1 kg and 3 kg have position vectors $$\widehat i + 2\widehat j + \widehat k$$ and $$ - 3\widehat i - 2\widehat j + \widehat k$$, respectively. The center of mass of this system has a position vector :
36
A black body at 227oC radiates heat at the rate of 7 cals/cm2s. At a temperature of 727oC, the rate of heat radiated in the same units will be
37
The two ends of a rod of length L and a uniform cross-sectional area A are Kept at two temperatures T1 and T2 (T1 > T2). The rate of heat transfer, $${{dQ} \over {dt}}$$ through the rod in a steady state is given by :
38
The internal energy change in a system that has absorbed 2 kcal of heat and done 500 J of work is
39
In thermodynamic processes which of the following statements is not true ?
40
A simple pendulum performs simple harmonic motion about x = 0 with an amplitude a and time period T. The speed of the pendulum at x = a/2 will be
41
Which one of the following equations of motion represents simple harmonic motion ?
where k, k0, k1 and a are all positive.
where k, k0, k1 and a are all positive.
42
Each of the two strings of length 51.6 cm and 49.1 cm are tensioned separately by 20 N force. Mass per unit length of both the strings is same and equal to 1 g/m. When both the strings vibrate simultaneously the number of beats is
43
A wave in a string has an amplitude of 2 cm. The wave travels in the +ve direction of x axis with a speed of 128 m/s. and it is noted that 5 complete waves fit in 4m length of the string. The equation describing the wave is
44
Three concentric spherical shells have radii a, b and c (a < b < c) anf have surface charge densities $$\sigma $$, $$-$$$$\sigma $$ and $$\sigma $$ respectively. If VA, VB and VC denote the potentials of the three shells, then, for c = a + b, we have
45
Three capacitors each of capacitance C and of breakdown voltage V are joined in series. The capacitance and breakdown voltages of the combination will be
46
If the dimensions of a physical quantity are given by MaLbTc, then the physical quantity will be