AIPMT 2005
Paper was held on
Sun, May 1, 2005 10:00 AM
Biology
1
An acromian process is characteristically found in the
2
Photosynthesis in C4 plants is relatively less limited by atmospheric CO2 levels because
3
Photosynthetic Active Radiation (PAR) has the following range of wavelengths.
4
During which stage in the complete oxidation of glucose are the greatest number of ATP molecules formed from ADP?
5
The ability of the Venus Flytrap to capture insects is due to
6
The net pressure gradient that causes the fluid to filter out of the glomeruli into the capsule is
7
In ornithine cycle, which of the following wastes are removed from the blood?
8
A person is undergoing prolonged fasting. His urine will be found to contain abnormal quantities of
9
As compared to a C3-plant, how many additional molecules of ATP are needed for net production of one molecule of hexose sugar by C4-plants?
10
Which of the following pairs is correctly matched?
11
Which one of the following is the example of the action of the autonomous nervous system?
12
Parkinson's disease (characterized by tremors and progressive rigidity of limbs) is caused by degeneration of brain neurons that are involved in movement control and make use of neurotransmitter
13
In a man, abducens nerve is injured. Which one of the following functions will be affected?
14
In a type of apomixis known as advice embryony, embryos develop directly from the
15
Through which cell of the embryo sac, does the pollen tube enter the embryo sac?
16
Which one of the following represents an ovule, where the embryo sac becomes horse-shoe shaped and the funiculus and micropyle are close to each other?
17
Grey crescent is the area -
18
If mammalian ovum fails to get fertilized, which
one of the following is unlikely
19
In order to find out the different types of
gametes produced by a pea plants having the
genotype AaBb, it should be crossed to a plant
with the genotype -
20
G-6-P dehydrogenase deficiency is associated
with haemolysis of -
21
Which one of the following characters is not typical of the class mammalia?
22
All of the following statements concerning the actinomycetous filamentous soil bacterium Frankia are correct except that Frankia
23
For retting of jute the fermenting microbe used is
24
Basophilic prokaryotes
25
There exists a close association between the alga and the fungus within a lichen. The fungus
26
Auxospores and hormogonia are formed, respectively, by
27
Match items in column $${\rm I}$$ with those in column $${\rm I}$$$${\rm I}$$.
Select the correct answer from the following.
| Column $${\rm I}$$ | Column $${\rm I}$$$${\rm I}$$ | ||
|---|---|---|---|
| (A) | Peritrichous flagellation | (J) | Ginkgo |
| (B) | Living fossil | (K) | Macrocystis |
| (C) | Rhizophore | (L) | Eschericha coli |
| (D) | Smallest flowering plant | (M) | Selaginella |
| (E) | Largest perennial alga | (N) | Wolffia |
Select the correct answer from the following.
28
Ectophloic siphonostele is found in
29
Top-shaped multiciliate male gametes and the nature seed which bears only one embryo with two cotyledons, are characterised features of
30
In contrast to annelids the platyhelminthes show
31
From the following statements select the wrong one
32
Which of the following unicellular organisms has a macronucleus for trophic function and one or more micronuclei for reproduction?
33
A man and a woman, who do not show any
apparent signs of a certain inherited disease,
have seven children (2 daughter and 5 sons).
Three of the sons suffer from the given disease
but none of the daughters are affected. Which of
the following mode of inheritance do you
suggest for this disease
34
Why is vivipary an undesirable character for annual crop plants?
35
Which of the following represents the edible part of the fruit of litchi?
36
Enzymes, vitamins and hormones can be classified into a single category of biological chemicals, because all of these
37
The catalytic efficiency of two different enzymes can be compared by the
38
Which one of the following statements regarding enzyme inhibition is correct?
39
Which of the following is the simplest amino acid?
40
Nucleotides are building blocks of nucleic acids. Each nucleotide is a composite molecule formed by
41
Carbohydrates, the most abundant biomolescule on earth, are produced by
42
At what stage of the cell cycle are histone proteins synthesized in a eukaryotic cell?
43
Bacillus thuringiensis (Bt) strains have been
used for designing novel -
44
Golden rice is a transgenic crop of the future
with the following improved trait -
45
Production of a human protein in bacteria by
genetic engineering is possible because
46
More than 70% of world's fresh water is
contained in –
47
At which latitude, heat gain through insolation
approximately equals heat loss through
terrestrial radiation -
48
Which one of the following pairs in
mismatched ?
49
Animals have the innate ability to escape from
predation. Examples for the same are given
below. Select the incorrect example
50
Biodiversity Act of India was passed by the
Parliament in the year -
51
According to IUCN Red List, what is the status
of Red Panda (Ailurus fulgens) ?
52
The world's highly prized wool yielding
'Pashmina' breed is -
53
In a woody dicotyledonous tree, which of the
following parts will mainly consist of primary
tissues ?
54
Four healthy people in their twenties got
involved in injuries resulting in damage and
death of a few cell of the following. Which of the
cells are least likely to be replaced by new cells -
55
A student wishes to study the cell structure under
a light microscope having 10X eyepiece and 45X
objective. He should illuminate the object by
which one of the following colours of light so as
get the best possible resolution ?
56
Chlorophyll in chloroplasts is located in
57
Centromere is required for -
58
Chemiosmotic theory of ATP synthesis in the
chloroplasts and mitochondria is based on
59
According to widely accepted "fluid mosaic
model" cell membranes are semi-fluid, where
lipids and integral proteins can diffuse randomly.
In recent years, this model has been modified in
several respects. In this regard, which of the
following statements are incorrect ?
60
The main organelle involved in modification and
routing of newly synthesized proteins to their
destinations is -
61
A women with 47 chromosomes due to three
copies of chromosome 21 is characterized by
62
De Vries gave his mutation theory on organic
evolution while working on -
63
A women with 47 chromosomes due to three
copies of chromosome 21 is characterized by -
64
Haemophilia is more commonly seen in human
males than in human females because -
65
A woman with normal vision, but whose father
was colour bind, marries a colour blind man.
Suppose that the fourth child of this couple was
a boy. This boy -
66
Which of the following is not a hereditary
disease ?
67
E. coli cells with a mustard z gene of the lac
operon cannot grow in medium containing only
lactose as the source of energy because -
68
Telomerase is an enzyme which is a -
69
Using imprints from a plate with complete
medium and carrying bacterial colonies, you
can select streptomycin resistant mutants and
prove that such mutations do not originates as
adaptation. These imprints need to be used -
70
Which one of the following hydrolyses internal
phosphodiester bonds in a polynucleotide chain ?
71
Protein synthesis in an animal cell occurs -
72
Which one of the following makes use of RNA
as a template to synthesize DNA -
73
During transcription holoenzyme RNA
polymerase binds to a DNA sequence and the
DNA assumes a saddle like structure at the point.
What is the sequence called ?
74
One of the most important functions of botanical gardens is that
75
Which one of the following experiments
suggests that simplest living organisms could
not have originated spontaneously from non-living matter ?
76
There are two opposing views about origin of
modern man. According to one view Homo
erectus in Asia were the ancestors of modern
man. A study of variation of DNA however
suggested African origin of modern man. What
kind of observation of DNA variation could
suggest this ?
77
Which one of the following phenomena supports
Darwin's concept of natural selection in organic
evolution ?
78
Which of the following is not true for a species ?
79
At a particular locus, frequency of 'A' allele is
0.6 and that of 'a' is 0.4. What would be the
frequency of heterozygotes in a random mating
population at equilibrium ?
80
Which of the following is the relatively most
accurate method for dating of fossils ?
81
Damage to thymus in a child may lead to
82
Which one of the following depresses brain
activity and produces feelings of calmness,
relaxation and drowsiness ?
Chemistry
1
Which one of the following orders correctly represents the increasing acid strengths of the given acids?
2
The energy of second Bohr orbit of the hydrogen atom is $$-$$328 kJ mol$$-$$1; hence the energy of fourth Bohr orbit would be
3
For a first order reaction A $$ \to $$ B the reaction rate a reactant concentration of 0.01 M is found to be 2.0 $$ \times $$ 10$$-$$5 mol L$$-$$1 s$$-$$1. The half-life period of the reaction is
4
The cell membranes are mainly composed of
5
Which functional group participates in disulphide bond formation in proteins?
6
The mole fraction of the solute in one molal aqueous solution is
7
The vapour pressure of two liquids P and Q are 80 and 60 torr, respectively. The total vapour pressure of solution obtained by mixing 3 mole of P and 2 mole of Q would be
8
A solution has a 1 : 4 mole ratio of pentane to hexane. The vapour pressures of the pure hydrocarbons at 20oC are 440 mm Hg for pentane and 120 mm Hg for hexane. The mole fraction of pentane in the vapour phase would be
9
A solution of urea (mol. mass 56 g mol$$-$$1) boils at 100.18oC at the atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol$$-$$1 respectively, the above solution will freeze at
10
4.5 g of aluminium (at. mass 27 amu) is deposited at cathode from Al3+ solution by a certain quantity of electric charge. The volume of hydrogen produced at STP from H ions in solution by the same quantity of electric charge will be
11
The mass of carbon anode consumed (giving only carbon dioxide) in the production of 270 kg of aluminium metal from bauxite by the Hall process is
(Atomic mass : Al = 27)
(Atomic mass : Al = 27)
12
The rate of reaction between two reactions A and B decreases by a factor of 4 if the concentration of reactant B is doubled. The order of this reaction with respect to reactant B is
13
Aniline in a set of reactions yielded a product D.

The structure of the product D would be

The structure of the product D would be
14
Which one of the following oxides is expected to exhibit paramagnetic behaviour?
15
Which of the following would have a permanent dipole moment?
16
What is the correct relationship between the pH of isomolar solutions of sodium oxide, Na2O (pH1), sodium sulphide, Na2S (pH2), sodium srlenide, Na2Se (pH3) and sodium telluride Na2Te (pH4)?
17
More number of oxidation states are exhibited by the actinoids than by the lanthanoids. The main reason for this is
18
The number of moles of KMnO4 reduced by one mole of KI in alkaline medium is
19
The aqueous solution containing which one of the following ions will be colourless?
(Atomic number : Sc = 21, Fe = 26, Ti = 22, Mn = 25)
(Atomic number : Sc = 21, Fe = 26, Ti = 22, Mn = 25)
20
Four successive members of the first row transition elements are listed below with their atomic numbers. Which one of them is expected to have the highest third ionisation enthalpy?
21
A nuclide of an alkaline earth metal undergoes radioactive decay by emission of the periodic table to which the resulting daughter element would belong is
22
Which one of the following is an inner orbital complex as well as diamagnetic in behaviour?
23
Which one of the following is expected to exhibit optical isomerism? (en = ethylenediamine)
24
Which of the following undergoes nucleophilic substitution exclusively by SN1 mechanism?
25
Which one of the following arrangements represents the correct order of electron gain enthalpy (with negative sign) of the given atomic species ?
26
The correct order in which the O $$-$$ O bond length increases in the following is
27
Which of the following molecules has trigonal planar geometry?
28
The surface tension of which of the following liquid is maximum ?
29
Which of the following pairs of a chemical reaction is certain to result in a spontaneous reaction ?
30
A reaction occurs spontaneously if
31
The absolute enthalpy of neutralisation of the reaction :
Mg(O)(s) + 2HCl(aq) $$ \to $$ MgCl2(aq) + H2O(l) will be
Mg(O)(s) + 2HCl(aq) $$ \to $$ MgCl2(aq) + H2O(l) will be
32
H2S gas when passed through a solution of cations containing HCl precipitates the cations of second group of qualitative analysis but not those belonging to the fourth group. It is because
33
At 25oC, the dissociation constant of a base, BOH, is 1.0 $$ \times $$ 10$$-$$12. The concentration of hydroxyl ions in 0.01 M aqueous solution of the base would be
34
Equilibrium constants K1 and K2 for the following equilibriam:

are related as

are related as
35
Which one of the following pairs represents stereoisomerism?
36
Which amongst the following is the most stable carbocation?
37
The chirality of the compound


38
Names of some compounds are given. Which one is not in IUPAC system?
39
The best method for the separation of naphthalene and benzoic acid from their mixture is
40
Products of the following reaction :


41
Which one of the following alkenes will react faster with H2 under catalytic hydrogenation conditions ?
42
Which one of the following compounds is most acidic?
43
The correct sequence of increasing covalent character is represented by
44
The major organic product formed from the following reaction :

is

is
45
In a set of reactions acetic acid yielded a product D.

The structure of D would be

The structure of D would be
46
Electrolytic reduction of nitrobenzene in weakly acidic medium gives
Physics
1
In the reaction $${}_1^2$$H + $${}_1^3$$H $$ \to $$ $${}_2^4$$He + $${}_0^1$$n, if the binding
energies of $${}_1^2$$ H, $${}_1^3$$H and $${}_2^4$$He are respectively a, b and c (in MeV),
then the energy (in MeV) released in this reaction is
energies of $${}_1^2$$ H, $${}_1^3$$H and $${}_2^4$$He are respectively a, b and c (in MeV),
then the energy (in MeV) released in this reaction is
2
A 5-ampere fuse wire can withstand a maximum power of 1 watt in the circuit. The resistance of the fuse wire is
3
An electron moves in a circular orbit with a uniform speed v. It producess a magnetic field B at the centre of the circle. The radius of the circle is proportional to
4
A very long straight wire carries a current $$I$$. At the instant when a charge +Q at point P has velocity $$\overrightarrow v $$, as shown, the force on the charge is


5
A coil in the shape of an equilateral triangle of side $$l$$ is suspended between the pole pieces of a permanent magnet such that $$\overrightarrow B $$ is in plane of the coil. If due to a current i in the triangle a torque $$\tau $$ acts on it, the side $$l$$ of the triangle is
6
If the magnetic dipole moment of an atom of diamagnetic material, paramagnetic material and ferromagnetic material are denoted by $$\mu $$d, $$\mu $$p and $$\mu $$f respectively, then
7
As a result of change in the magnetic flux linked to the closed loop as shown in the figure, an e.m.f. $$V$$ volt is induced in the loop. The work done (joule) in taking a charge Q coulomb once along the loop is


8
In a circuit L, C and R are connected in series with an alternating voltage source of frquency $$f.$$ The current leads the voltage by 45o. The value of C is
9
If $$\lambda $$v, $$\lambda $$x and $$\lambda $$m represent the wavelengths of visible light, X-rays and microwaves respectively, then
10
The angular resolution of a 10 cm diameter telescope at a wavelength of 5000 $$\mathop A\limits^ \circ $$ is of the order of
11
The work functions for metals A, B and C are respectively 1.92 eV, 2.0 eV and 5 eV. According to Einstein's equation the metals which will emit photoelectrons for a radiation of wavelength 4100 $$\mathop A\limits^ \circ $$ is/are
12
A photosensitive metallic surface has work function, h$$\upsilon $$0. If photons of energy $$2h{\upsilon _0}$$ fall on this surface, the electrons come out with a maximum velocity of 4 $$ \times $$ 106 m/s. When the photon energy is increased to 5 h$$\upsilon $$0, then maximum velocity of photoelectrons will be
13
For the network shown in the figure the value of the current $$i$$ is
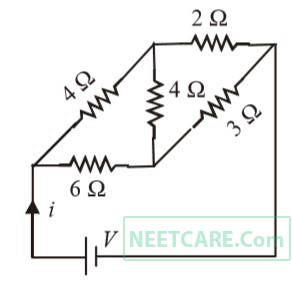

14
The total energy of an electron in the first excited state of hydrogen atom is about $$-$$ 3.4 eV. Its kinetic energy in this state is
15
In any fission process the ratio
mass of fission products
mass of parent nucleus
is 16
Fission of nuclei is possible because the binding energy per nucleon in them
17
Energy levels A, B and C of a certain atom corresponding to increasing values of energy i.e. EA < EB < EC. If $$\lambda $$1, $$\lambda $$2 and $$\lambda $$3 are wavelengths of radioations corresponding to transitions C to B, B to A and C to A respectively, which of the following relations is correct?
18
Choose the only false statement from the following.
19
Application of a forward bias to a p-n junction
20
Zener diode is used for
21
Carbon, silicon and germanium atoms have four valence electrons each. Their valence and conduction bands are separated by energy band gaps represented by (Eg)C, (Eg)si and (Eg)Ge respectively. Which one of the following relationships is true in their case?
22
Copper has face centered cubic (fcc) lattice with interatomic spacing equal to 2.54 $$\mathop A\limits^ \circ $$. The value of lattice constant for this lattice is
23
In a p-n junction photo cell, the value of the photo-electromotive force profuced by monochromatic light is proportional to
24
For a satellite moving in an orbit around the earth, the ratio of kinetic energy to potential energy is
25
A ball is thrown vertically upward. It has a speed of 10 m/sec when it has reached one half of its maximum height. How high does the ball rise?
(Take g = 10 m/s2.)
(Take g = 10 m/s2.)
26
The displacement x of a particle varies with time t as x = ae$$-$$at + be$$\beta $$t, where a, b, $$\alpha $$ and $$\beta $$ are positive constants. The velocity of the particle will
27
Two boys are standing at the ends A and B of a ground where AB = a. The boy at B starts running in a direction perpendicular to AB with velocity v1. The boy at A starts running simultaneously with velocity v and catches the other in a time t, where t is
28
A stone tied to the end of a string of 1 m long is whirled in a horizontal circle with a constant speed. If the stone makes 22 revoluations in 44 seconds, what is the magnitude and direction of acceleration of the stone ?
29
If the angle between the vectors $$\overrightarrow A $$ and $$\overrightarrow B $$ is $$\theta $$, the value of the product $$\left( {\overrightarrow B \times \overrightarrow A } \right).\overrightarrow A $$ is equal to
30
If a vector $$2\widehat i + 3\widehat j + 8\widehat k$$ is perpendicular to the vector $$4\widehat j - 4\widehat i + \alpha \widehat k,$$ then the value of $$\alpha $$ is
31
A bomb of mass 30 kg at rest explodes into two pieces of masses 18 kg and 12 kg. The velocity of 18 kg mass is 6 m s$$-$$1. The kinetic energy of the other mass is :
32
A force F acting

on an object varies with distance x as shown here. The force is in N and x in m. The work done by the force in moving the object from x = 0 to x = 6 m is

on an object varies with distance x as shown here. The force is in N and x in m. The work done by the force in moving the object from x = 0 to x = 6 m is
33
A drum of radius R and mass M, rolls down without slipping along an inclined plane of angle $$\theta $$. The frictional force
34
The moment of inertia of a uniform circular disc of radius R and mass M about an axis passing from the edge of the disc and normal to the disc is
35
Two bodies have their moments of inertia I and 2I respectively about their axis of rotation. If their kinetic energies of rotation are equal, their angular velocity will be in the ratio
36
Imagine a new planet having the same density as that of earth but it is 3 times bigger than the earth in size. If the acceleration due to gravity on the surface of earth is g and that on the surface of the new planet is g', then
37
Which of the following rods, (given radius r and length $$l$$) each made of the same material and whose ends are maintained at the same temperature will conduct most heat ?
38
An ideal gas heat engine operates in Carnot cycle between 227oC and 127oC. It absorbs 6 $$ \times $$ 104 cal of heat at higher temperature. Amount of heat converted to work is
39
Which of the following processes is reversible?
40
The circular motion of a particle with constant speed is
41
A point source emits sound equally in all directions in a non-absorbing medium. Two points P and Q are at distances of 2 m and 3 m respectively from the source. The ratio of the intensities of the waves at P and Q is
42
A network of four capacitors of capacity equal to C1 = C, C2 = 2C, C3 = 3C and C4 = 4C are connected to a battery as shown in the figure. The ratio of the charges on C2 and C4 is


43
As per the diagram a point charge +q is placed at the origin O. Work done in taking another point charge $$-$$Q from the point A [coordinates (0, $$a$$)] to another point B


44
Two charges q1 and q2 are placed 30 cm apart, as shown in the figure. A third charge q3 is moved along the arc of a circle of radius 40 cm from C to D.
The change in the potential energy of the system is $${{{q_3}} \over {4\pi {\varepsilon _0}}}$$ where k is

The change in the potential energy of the system is $${{{q_3}} \over {4\pi {\varepsilon _0}}}$$ where k is

45
Two batteries, one of emf 18 volts and internal resistance 2 $$\Omega $$ and the other of emf 12 volts and internal resistance 1 $$\Omega $$, are connected as shown. The voltmeter V will record a reading of
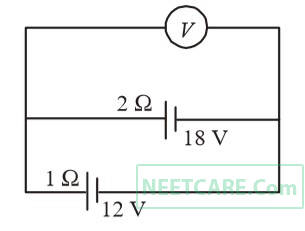

46
When a wire of uniform cross-section $$a$$ length $$l$$ and resistance R is bent into a complete circle. resistance between any two of diametrically opposite points will be
47
The ratio of the dimensions of Planck's constant and that of moment of inertia is the dimensions of