AIPMT 2011 Mains
Paper was held on
Sun, May 15, 2011 10:00 AM
Biology
1
Bulk of carbon dioxide (CO2) released from body tissues into the blood is present as
2
Common cold is not cured by antibiotics
because it is :
3
Which one of the following options gives the
correct matching of a disease with its causative
organism and mode of infection ?
4
The unequivocal proof of DNA as the genetic
material came from the studies on a :
5
Test cross in plants or in Drosophila involves
crossing :
6
Which one of the following conditions of the
zygotic cell would lead to the birth of a normal
human female child ?
7
The technique called gamete intrafallopian
transfer (GIFT) is recommended for those
females :
8
What happens during fertilization in humans
after many sperms reach close to the ovum?
9
About which day in a normal human menstrual
cycle does rapid secretion of LH (popularly
called LH-surge) normally occurs?
10
What is common between vegetative reproduction and apomixts?
11
In angiosperms, functional megaspore develops into
12
The 24 hour (diurnal) rhythm of our body such as the sleep-wake cycle is regulated by the hormone
13
The type of muscles present in our
14
Uricotelic mode of excreting nitrogenous wastes is found in
15
Given below is the ECG of a normal human. Which one of its components is correctly interpreted below?


16
Read the following statement having two blanks
(A and B) :
''A drug used for ______ (A)___ patients is obtained from a species of the organism ______ (B)___ ''.
The one correct option for the two blanks is :
''A drug used for ______ (A)___ patients is obtained from a species of the organism ______ (B)___ ''.
The one correct option for the two blanks is :
17
Which one of the following is a possibility for most of us in regard to breathing, by making a conscious effort?
18
In mitochondria, protons accumulate in the
19
In kranz anatomy, the bundle sheath cells have
20
At metaphase, chromosomes are attached to the spindle fibres by their
21
Which one of the following pairs is wrongly matched while the remaining three are correct?
22
Sweet potato is homologous to
23
Whorled, simple leaves with reticulate venation are present in
24
Which one of the following statements is totally wrong about the occurrence of notochord, while the other three are correct?
25
Consider the following four statements (A$$-$$D) related to the common frog Rana tigrina, and select the correct option starting which ones are true (T) and which ones are false (F). Statements:
A. On dry land it would die due to lack of O2 if its mouth is forcibly kept closed for a few days.
B. It has four-chambered heart.
C. On dry land it turns uricotelic from ureotelic.
D. Its life-history is carried out in pond water.
A. On dry land it would die due to lack of O2 if its mouth is forcibly kept closed for a few days.
B. It has four-chambered heart.
C. On dry land it turns uricotelic from ureotelic.
D. Its life-history is carried out in pond water.
26
Ureters act as urinogenital ducts in
27
Some vascular bundles are described as open
because these :
28
Which one of the following is essential for
photolysis of water?
29
The figures (A – D) show four animals. Select
the correct option with respect to a common
characteristic of two of these animals.


30
Which one of the following figures represents
the placentation in Dianthus?
31
Examine the figure given below and select the
correct option giving all the four parts (A, B, C
and D) rightly identified.


32
The figure below shows the structure of a
mitochondrion with its four parts labelled (A),
(B), (C). and (D) Select the part correctly
matched with its function. –


33
Which one of the following is not considered as
a part of the endomembrane system ?
34
Which one of the following structure in pheretima
is correctly matched with its function ?
35
The cells lining the blood vessels belongs to the
category of :
36
Frogs differ from humans in possessing :
37
Function of companion cells is :
38
Consider the following four statements whether they are correct or wrong.
A. The sporophyte in liverworts is more eleborate than that in mosses.
B. Salvinia is heterosporous.
C. The life-cycle in all seed-bearing plants is diplontic.
D. In Pinus male and female cones are borne on different trees.
The two wrong statements together are
A. The sporophyte in liverworts is more eleborate than that in mosses.
B. Salvinia is heterosporous.
C. The life-cycle in all seed-bearing plants is diplontic.
D. In Pinus male and female cones are borne on different trees.
The two wrong statements together are
39
Biodiversity of a geographical region represents :
40
Consider the following statements (A)-(D) each
with one or two blanks :
(A) Bears go into _____ (1) ____ during winter to _____(2)____ cold weather.
(B) A conical age pyramid with a broad base represents ____(3)_____ human population.
(C) A wasp pollinating a fig flower is an example of _____ (4) _____ .
(D) An area with high levels of species richness is known as ____(5)_____.
Which of the following options, gives the correct fill ups for the respective blank numbers from (1) to (5) in the statements ?
(A) Bears go into _____ (1) ____ during winter to _____(2)____ cold weather.
(B) A conical age pyramid with a broad base represents ____(3)_____ human population.
(C) A wasp pollinating a fig flower is an example of _____ (4) _____ .
(D) An area with high levels of species richness is known as ____(5)_____.
Which of the following options, gives the correct fill ups for the respective blank numbers from (1) to (5) in the statements ?
41
The breakdown of detritus into smaller particles
by earthworm is a process called
42
Both, hydrarch and xerarch successions lead to :
43
Which one of the following animals may occupy
more than one trophic levels in the same
ecosystem at the same time ?
44
The logistic population growth is expressed by
the equation :
45
Read the following four statements (A-D) about
certain mistakes in two of them :
(A) The first transgenic buffalo, Rosie produced milk which was human alpha-lactalbumin enriched.
(B) Restriction enzymes are used in isolation of DNA from other macro-molecules
(C) Downstream processing is one of the steps of R-DNA technology
(D) Disarmed pathogen vectors are also used in transfer of R-DNA into the host
Which are the two statements having mistakes ?
(A) The first transgenic buffalo, Rosie produced milk which was human alpha-lactalbumin enriched.
(B) Restriction enzymes are used in isolation of DNA from other macro-molecules
(C) Downstream processing is one of the steps of R-DNA technology
(D) Disarmed pathogen vectors are also used in transfer of R-DNA into the host
Which are the two statements having mistakes ?
46
Silencing of mRNA has been used in producing
transgenic plants resistant to :
47
Bacillus thuringiensis forms protein crystals
which contain insecticidal protein. This protein :
48
Which one of the following techniques made it
possible to genetically engineer living organisms ?
49
Consider the following statements (A-D) about
organic farming :
(A) Utilizes genetically modified crops like Bt cotton
(B) Uses only naturally produced inputs like compost
(C) Does not use pesticides and urea
(D) Produces vegetables rich in vitamins and minerals
Which of the above statements are correct ?
(A) Utilizes genetically modified crops like Bt cotton
(B) Uses only naturally produced inputs like compost
(C) Does not use pesticides and urea
(D) Produces vegetables rich in vitamins and minerals
Which of the above statements are correct ?
50
Which one of the following is a wrong matching
of a microbe and its industrial product, while the
remaining three are correct ?:
51
Selaginella and Salvinia are considered to represent a significant step toward evolution of seed habit because
52
The pathogen Microsporum responsible for ringworm disease in humans belongs to the same kingdom of organisms as that of
53
Which one of the following aspects is in exclusive characteristics of living things?
Chemistry
1
Which of the following complex compounds will
exhibit highest paramagnetic behaviour?
(At. No. Ti = 22, Cr = 24, Co = 27, Zn = 30)
(At. No. Ti = 22, Cr = 24, Co = 27, Zn = 30)
2
Which of the following carbonyls will have the
strongest C–O bond ?
3
The pairs of species of oxygen and their
magnetic behaviours are noted below. Which of
the following presents the correct description?
4
Which has the maximum number of molecules among the following ?
5
According to the Bohr theory, which of the following transitions in the hydrogen atom will give rise to the least energetic photon ?
6
Which of the following is not a fat soluble vitamin?
7
Which of the following oxide is amphoteric?
8
Which of the following statements is incorrect?
9
The unit of rate constant for a zero order reaction is
10
The half-life of a substance in a certain enzyme-catalysed reaction is 138 s. The time required for the concentration of the substance to fall from 1.28 mg L$$-$$1 to 0.04 mg L$$-$$1 is
11
The rate of the reaction : 2N2O5 $$ \to $$ 4NO2 + O2
can be written in three ways.
$${{ - d\left[ {{N_2}{O_5}} \right]} \over {dt}} = k\left[ {{N_2}{O_5}} \right]$$
$${{d\left[ {N{O_2}} \right]} \over {dt}} = k'\left[ {{N_2}{O_5}} \right];\,\,$$ $${{d\left[ {{O_2}} \right]} \over {dt}} = k''\left[ {{N_2}{O_5}} \right]$$
The relationship between k and k' and between k and k'' are
can be written in three ways.
$${{ - d\left[ {{N_2}{O_5}} \right]} \over {dt}} = k\left[ {{N_2}{O_5}} \right]$$
$${{d\left[ {N{O_2}} \right]} \over {dt}} = k'\left[ {{N_2}{O_5}} \right];\,\,$$ $${{d\left[ {{O_2}} \right]} \over {dt}} = k''\left[ {{N_2}{O_5}} \right]$$
The relationship between k and k' and between k and k'' are
12
A solution contains Fe2+, Fe3+ and I$$-$$ ions. This solution was treated with iodine at 35oC. Eo for Fe3+/Fe2+ is + 0.77 V and Eo for I2/2I$$-$$ = 0.536 V.
The favourable redox reaction is
The favourable redox reaction is
13
A 0.1 molal aqueous solution of a weak acid is 30% ionized. If Kf for water is 1.86oC/m, the freezing point of the solution will be
14
200 mL of an aqueous solution of a protein contains its 1.26 g. The osmotic pressure of this solution at 300 K is found to be 2.57 $$ \times $$ 10$$-$$3 bar. The molar mass of protein will be (R = 0.083 L bar mol$$-$$1 K$$-$$1)
15
Which of the statements about ''Denaturation'' given below are correct?
(1) Denaturation of proteins causes loss of secondary and tertiary structures of the protein.
(2) Denaturation leads to the conversion of double strand of DND into single strand.
(3) Denaturation affects primary structure which gets distorted.
(1) Denaturation of proteins causes loss of secondary and tertiary structures of the protein.
(2) Denaturation leads to the conversion of double strand of DND into single strand.
(3) Denaturation affects primary structure which gets distorted.
16
What of the following compounds is most basic?
17
The order of reactivity of phenyl magnesium bromide (PhMgBr) with the following compounds:


18
An organic compound A on treatment with NH3 gives B, which on heating gives C. C when treated with Br2 in the presence of KOH produces ethyl amine. Compound A is
19
Match the compounds given in List-I with List-II and select the suitable option using the code given below.
| List-I |
List-II |
||
|---|---|---|---|
| (A) | Benzaldehyde | (i) | Phenolphthalein |
| (B) | Phthalic anhydride | (ii) | Benzoin condensation |
| (C) | Phenyl benzoate | (iii) | Oil of wintergreen |
| (D) | Methyl salicylate | (iv) | Fries rearrangement |
20
Consider the reactions.

The mechanisms of reactions (i) and (ii) are respectively

The mechanisms of reactions (i) and (ii) are respectively
21
The IUPAC name of the following compound is


22
Which of the following compounds undergoes nucleophilic substitution reaction most easily?
23
In qualitative analysis, the metals of group I can be separated from other ions by precipitating them as chloride salts. A solution initially contains Ag+ and pb2+ at a concentration of 0.10 M. Aqueous HCl is added to this solution until the Cl$$-$$ concentration is 0.10 M. What will the concentrations of Ag+ and Pb2+ be at equilibrium ?
(Ksp for AgCl = 1.8 $$ \times $$ 10$$-$$10, Ksp for PbCl2 = 1.7 $$ \times $$ 10$$-$$5)
(Ksp for AgCl = 1.8 $$ \times $$ 10$$-$$10, Ksp for PbCl2 = 1.7 $$ \times $$ 10$$-$$5)
24
Consider the following processes :
For B + D $$ \to $$ E + 2C, $$\Delta $$H will be
| H (kJ/mol) | |
|---|---|
| 1/2A $$ \to $$ B | +150 |
| 3B $$ \to $$ 2C + D | -125 |
| E + A $$ \to $$ 2D | +350 |
For B + D $$ \to $$ E + 2C, $$\Delta $$H will be
25
Which of the following structures is the most preferred and hence of lowest energy for SO3?
26
What is the value of electron gain enthalpy of Na+ if IE1 of Na = 5.1 eV?
Physics
1
A thermocouple of negligible resistance
produces an e.m.f. of 40 µV/ºC in the linear range
of temperature. A galvanometer of resistance 10
ohm whose sensitivity is 1 µA/division, is
employed with the thermocouple. The smallest
value of temperature difference that can be
detected by the system will be
2
A galvanometer of resistance, G, is shunted by a resistance S ohm. To keep the main current in the circuit unchanged, the resistance to be put in series with the galvanometer is
3
Pure Si at 500 K has equal number of electron (ne) and hole (nh) concentrations of 1.5 $$ \times $$ 1016 m$$-$$3. Doping by indium increases nh to 4.5 $$ \times $$ 1022 m$$-$$3. The doped semiconductor is of
4
In the following figure, the diodes which are forward biased, are


5
A Zener diode, having breakdown voltage equal to 15 V, is used in a voltage regulator circuit shown in figure. The current through the diode is


6
Out of the following which one is not a possible energy for a photon to be emitted by hydrogen atom according to Bohr's atomic model?
7
An electron in the hydrogen atom jumps from excited state n to the ground state. The wavelength so emitted illuminates a photosensitive material having work function 2.75 eV. If the stopping potential of the photoelectron is 10 V, then the value of n is
8
The threshold frequency for a photosensitive metal is 3.3 $$ \times $$ 1014 Hz. If light of frequency 8.2 $$ \times $$ 1014 Hz is incident on this metal, the cut- off voltage for the photoelectron emission is nearly
9
A thin prism of angle 15o made of glass of refractive index $$\mu $$1 = 1.5 is combined with another prism of glass of refractive index $$\mu $$2 = 1.75. The combination of the prisms produces dispersion without deviation. The angle of the second prism should be
10
A converging beam of rays is incident on a diverging lens. Having passed through the lens the rays intersect at a point 15 cm from the lens on the opposite side. If the lens is removed the point where the rays meet will move 5 cm closer to the lens. The focal length of the lens is
11
A coil has resistance 30 ohm and inductive reactance 20 ohm at 50 Hz frequency. If an ac source, of 200 volt, 100 Hz, is connected across the coil, the current in the coil will be
12
The r.m.s. value of potential difference V shown in the figure is


13
A short bar magnet of magnetic moment 0.4 J T$$-$$1 is placed in a uniform magnetic field of 0.16 T. The magnet is in stable equilibrium when the potential energy is
14
A square loop, carrying a teady current $$I$$, is placed in a horizontal plane near a long straight conductor carrying a steady current $$I$$1 at a distance d from the conductor as shown in figure. The loop will experience


15
Charge q is uniformly spread on a thin ring of radius R. The ring rotates about its axis with a uniform frequency $$f$$ Hz. The magnitude of magnetic induction at the center of the ring is
16
In the circuit shown in the figure, if the potential at point A is taken to be zero, the potential at point B is
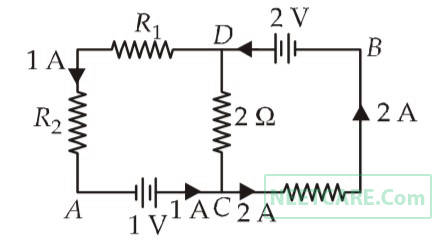

17
Three charges, each +q, are placed at the corners of an isosceles triangle ABC of sides BC and AC, 2$$a$$. D and E are the mid points of BC and CA. The work done in taking a charge Q from D to E is


18
The electric potential V at any point (x, y, z), all in metres in space is given by V = 4x2 volt. The electric field at the point (1, 0, 2) in volt/meter, is
19
Two identical piano wires, kept under the same tension T have a fundamental frequency of 600 Hz. The fractional increase in the tension of one of the wires which will lead to occurrence of 6 beats/s when both the wires oscillate together would be
20
Two particles are oscillating along two close parallel straight lines side by side, with the same frequency and amplitudes. They pass each other, moving in opposite directions when their displacement is half of the amplitude. The mean positions of the two particles lie on a straight line perpendicular to the paths of the two particles. The phase difference is
21
A mass of diatomic gas $$(\gamma = 1.4)$$ at a pressure of 2 atmospheres is compressed adiabatically so that its temperature rises from 27oC to 927oC. The pressure of the gas in the final state is
22
A particle of mass M is situated at the centre of a spherical shell of same mass and radius a. The magnitude of the gravitational potential at a point sutuated at a/2 distance from the centre, will be :
23
A particle of mass m is thrown upwards from the surface of the earth, with a velocity u. The mass and the radius of the earth are, respectively, M and R. G is gravitational constant and g is acceleration due to gravity on the surface of the earth. The minimum value of u so that the particle does not return back to earth, is
24
A small mass attached to a string rotates on a frictionless table top as shown. If the tension in the string is increased by pulling the string causing the radius of the circular motion to decrease by a factor of 2, the kinetic energy of the mass will


25
A mass m moving horizontally (along the x-axis) with velocity $$v$$ collides and sticks to a mass of 3m moving vertically upwards (along the y-axis) with velocity 2$$v$$. The final velocity of the combination is
26
A conveyor belt is moving at a constant speed of 2 ms$$-$$1. A box is gently dropped on it. The coefficient of friction between them is $$\mu $$ = 0.5. The distance that the box will move relativce to belt before coming to rest on it, taking g = 10 m s$$-$$2,, is
27
A projectile is fired at an angle of 45o with the horizontal. Elevation angle of the projectile at its highest point as seen from the point of projection, is
28
A particle covers half of its total distance with speed v1 and the rest half distance with speed v2. Its average speed during the complete journey is
29
The density of a material in CGS system of units is 4 g cm$$-$$3. In a system of units in which unit of length is 10 cm and unit of mass is 100 g, the value of density of material will be