AIPMT 2014
Paper was held on
Sun, May 4, 2014 10:00 AM
Biology
1
Which one of the following is a non - reducing carbohydrate?
2
During which phase(s) of cell cycle, amount of DNA in a cell remains at 4C level if the initial amount is denoted as 2C?
3
In 'S' phase of the cell cycle
4
The enzyme recombinase is required at which stage of meiosis?
5
In which one of the following processes CO2 is not released?
6
Dr. F. went noted that if coleoptile tips were removed and placed on agar for one hour, the agar would produce a bending when placed on one side of freshly-cut coleoptile stumps. Of what significance is this experiment?
7
Which one of the following growth regulators is known as 'stress hormone'?
8
A few normal seedlings of tomato were kept in a dark room. After a few days they were found to have become white -coloured like albinos. Which of the following terms will you use to describe them?
9
Select the option which is not correct with respect to enzyme action.
10
Approximately seventy percent of carbon-dioxide absorbed by the blood will be transported to the lungs
11
Person with blood group AB is considered as universal recipient because he has
12
How do parasympathetic neutral signals affect the working of the heart?
13
Which of the following causes an increase in sodium reabsorption in the distal convoluted tubule?
14
Stimulation of a muscle fiber by a motor neuron occurs at
15
Select the correct matching of the type of the joint with the example in human skeletal system.
16
Which one of the following statements is not correct?
17
Injury localized to the hypothalamus would most likely disrupt
18
Identify the hormone with its correct matching of source and function.
19
Flight-or-flight reactions cause activation of
20
Male gametophyte with least number of cells is present in
21
Five kingdom system of classification suggested by R.H. Whittaker is not based on
22
Which of the following shows coiled RNA strand and capsomeres?
23
Archaebacteria differ from eubacteria in
24
Which structures perform the function of mitochondria in bacteria?
25
Viruses have
26
The motile bacteria are able to move by
27
Anoxygenic photosynthesis is characteristic of
28
Which one of the following is wrong about Chara?
29
Which one of the following shows isogamy with non-flagellated gametes?
30
Which of the following is responsible for peat formation?
31
Which one of the following living organisms completely lacks a cell wall?
32
Select the taxon mentioned that represents both marine and fresh water species.
33
Planaria possesses high capacity of
34
A marine cartilaginous fish that can produce electric current is
35
Planaria possesses high capacity of
36
Placenta and pericarp are both edible portions in
37
An example of edible underground steam is
38
When the margins of sepals or petals overlap one another without any particular direction, the condition is termed as
39
Which one of the following statements is correct?
40
Given below is the representation of the extent of global diversity of invertebrates. What
groups the four portions (A-D) represent respectively?


41
Which vector can clone only a small fragment of DNA ?
42
Commonly used vectors for human genome sequencing are :
43
The first human hormone produced by recombinant DNA technology is :
44
Just as a person moving from Delhi to Shimla to escape the heat for the duration of hot
summer, thousands of migratory birds from Siberia and other extremely cold northern regions
move to:
45
Match the following and select the correct option:
| (a) Earthworm | (i) Pioneer species |
|---|---|
| (b) Succession | (ii) Detritivore |
| (c) Ecosystem service | (iii) Natality |
| (d) Population growth | (iv) Pollination |
46
Given below is a simplified model of phosphorus cycling in a terrestrial ecosystem with four
blanks (A-D). Identify the blanks.


47
If 20 J of energy is trapped at producer level, then how much energy will be available to
peacock as food in the following chain?
Plant → mice → snake → peacock
Plant → mice → snake → peacock
48
An example of ex situ conservation is :
49
A species facing extremely high risk of extinction in the immediate future is called :
50
The organization which publishes the Red List of species is :
51
An analysis of chromosomal DNA using the Southern hybridization technique does not use:
52
You are given a fairly old piece of dicot stem and a dicot root. Which of the following
anatomical structures will you use to distinguish between the two?
53
Tracheids differ from other tracheary elements in :
54
Choose the correctly matched pair:
55
Choose the correctly matched pair :
56
The solid linear cytoskeletal elements having a diameter of 6 nm and made up of a single type
of monomer are known as:
57
The osmotic expansion of a cell kept in water is chiefly regulated by:
58
Match the following and select the correct answer :
| (a) Centriole | (i) Infoldings in mitochondria |
|---|---|
| (b) Chlorophyll | (ii) Thylakoids |
| (c) Cristae | (iii) Nucleic acid |
| (d) Ribozymes | (iv) Basal body cilia or flagella |
59
Which of the following is a hormone releasing Intra Uterine Device (IUD)?
60
An aggregate fruit is one which develops from
61
Geitonogamy involves
62
Pollen tablets are available in the market for
63
Function of filiform apparatus is to
64
Non-albuminous seed is produced in
65
The shared terminal duct of the repoductive and urinary system in the human male is
66
The main function of mammalian corpus luteum is to produce
67
Select the correct option describing gonadotropin activity in a normal pregnant female.
68
Tubectomy is a method of sterilization in which :
69
Assisted reproductive technology, IVF involves transfer of :
70
Transformation was discovered by :
71
Which one of the following is wrongly matched?
72
Select the correct option ;
73
Which is the particular type of drug that is obtained from the plant whose one flowering
branch is shown below?


74
At which stage of HIV infection does one usually show symptoms of AIDS ?
75
To obtain virus−free healthy plants from a diseased one by tissue culture technique, which
part/parts of the diseased plant will be taken ?
76
Which one of the following fungi contains hallucinogens?
77
An alga which can be employed as food for human being is :
78
What gases are produced in anaerobic sludge digesters ?
Chemistry
1
Which of the following complexes is used to be as an anticancer agent?
2
Among the following complexes the one which shows zero crystal field stabilization energy (CFSE) is
3
Among the following sets of reactants which one produces anisole?
4
The pair of compounds that can exist together is
5
(I) H2O2 + O3 $$ \to $$ H2O + 2O2
(II) H2O2 + Ag2O $$ \to $$ 2Ag + H2O + O2
Role of Hydrogen peroxide in the above reactions is respectvely
(II) H2O2 + Ag2O $$ \to $$ 2Ag + H2O + O2
Role of Hydrogen peroxide in the above reactions is respectvely
6
In acidic medium, H2O2 changes Cr2O72- to CrO5 which has two
($$-$$O$$-$$O$$-$$) bonds. Oxidation state of Cr in CrO5 is
($$-$$O$$-$$O$$-$$) bonds. Oxidation state of Cr in CrO5 is
7
Which one is most reactive towards nucleophilic addition reaction?
8
In the following reaction, the product (A) is


9
Which of the following will be most stable diazonium salt RN2+X$$-$$?
10
Which of the following hormones is produced under the condition of stress which simulate glycogenlysis in the liver of human beings?
11
D(+)-glucose reacts with hydroxyl amine and yields an oxime. The structure of the oxime would be
12
Which of the following will not be soluble in sodium hydrogen carbonate?
13
Of the following 0.10 m aqueous solutions, which one will exhibit the largest freezing point depression?
14
The weight of silver (at. wt. = 108) displaced by a quantity of electricity which displaces 5600 mL of O2 at STP will be
15
When 0.1 mol MnO$$_4^{2 - }$$ is oxidised the quantity of electricity required to completely oxidise MnO$$_4^{2 - }$$ to MnO$$_4^ - $$ is
16
Acidity of diprotic acids in aqueous solutions increases in the order
17
Magnetic moment 2.83 BM is given by which of the following ions?
(At. nos. Ti = 22, Cr = 24, Mn = 25, Ni = 28)
(At. nos. Ti = 22, Cr = 24, Mn = 25, Ni = 28)
18
The reaction of aqueous KMnO4 with H2O2 in acidic conditions gives
19
Reason of lanthanoid contraction is
20
For the reaction, $${X_2}{O_{4\left( l \right)}}\,\, \to \,\,2X{O_{2(g)}}$$
$$\Delta $$U = 2.1 kcal, $$\Delta $$S = 20 cal K$$-$$1 at 300 K
Hence, G is
$$\Delta $$U = 2.1 kcal, $$\Delta $$S = 20 cal K$$-$$1 at 300 K
Hence, G is
21
When 22.4 liters of H2(g) is mixed with 11.2 litres of Cl2(g), each at S.T.P. the moles of HCl(g) formed is equal to
22
Equal masses of H2, O2 and methane have been taken in a container of volume V at temperature 27oC in identical conditions. The ratio of the volumes of gases H2 : O2 : methane would be
23
1.0 g of magnesium is burnt with 0.56 g O2 in a closed vessel, Which reactant is left in excess and how much ? (At. wt. Mg = 24, O = 16)
24
What is the maximum number of orbitals that can be identified with the following quantum numbers ?
n = 3, l = 1, m1 = 0
n = 3, l = 1, m1 = 0
25
Calculate the energy in joule corresponding to light of wavelength 45 nm. (Planck,s constant, h = 6.63 $$ \times $$ 10$$-$$34 J s, speed of light, c = 3 $$ \times $$ 108 m s$$-$$1)
26
Be2+ is isoelectronic with which of the following ions ?
27
Which of the following orders of ionic radii is correctly represented ?
28
Which of the following molecules has the maximum dipole moment ?
29
Which one of the following species has plane triangular shape ?
30
Which of the following statements is correct for the spontaneous adsorption of a gas?
31
Which of the following salts will give highest pH in water?
32
Using the Gibb's energy change, $$\Delta $$Go = +63.3 kJ, for the following reaction,
Ag2CO3(s) $$\rightleftharpoons$$ 2 Ag+(aq) + CO32$$-$$ (aq)
the Ksp of Ag2CO3(s) in water at 25oC is
(R = 8.314 J K$$-$$1 mol$$-$$1)
Ag2CO3(s) $$\rightleftharpoons$$ 2 Ag+(aq) + CO32$$-$$ (aq)
the Ksp of Ag2CO3(s) in water at 25oC is
(R = 8.314 J K$$-$$1 mol$$-$$1)
33
For a given exothermic reaction, Kp and K'p are the equilibrium constants at temperatures T1 and T2, respectively. Assuming that heat of reaction is constant in temperature range between T1 and T2, it is readily observed that
34
For the reversible reaction,
N2(g) + 3H2(g) $$\rightleftharpoons$$ 2NH3(g) + heat
The equilibrium shifts in forward direction
N2(g) + 3H2(g) $$\rightleftharpoons$$ 2NH3(g) + heat
The equilibrium shifts in forward direction
35
In the Kjeldahl's method for estimation of nitrogen present in a soil sample, ammonia evolved from 0.75 g of sample neutralized 10 mL of 1 M H2SO4. The percentage of nitrogen in the soil is
36
What products are formed when the following compound is treated with Br2 in the presence of FeBr3?


37
Identify Z in the sequence of reactions :


38
Which of the following organic compounds has same hybridization as its combustion product (CO2)?
39
Which of the following compounds will undergo racemisation when solution of KOH hydrolyses?


Physics
1
Light with an energy flux of 25 $$ \times $$ 104 W m$$-$$2 falls on a perfectly reflecting surface at normal incidence. If the surface area is 15 cm2, the average force exerted on the surface is
2
A conducting sphere of radius R is given a charge Q. The electric potential and the electric field at the centre of the sphere rrespectively are
3
Two thin dielectric slabs of dielectric constants K1 and K2(K1 < K2) are inserted between plates of a parallel plate capacitor, as shown in the figure. The variation of electric field E between the plates with distance d as measured from plate P is correctly shown by


4
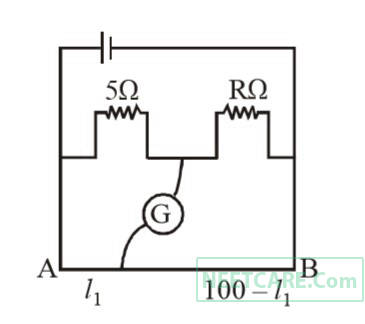
The resistances in the two arms of the meter bridge are 5 $$\Omega $$ and R$$\Omega $$ respectively. When the resistance R is shunted with an equal resistance, the new balance point is at 1.6$$l$$1. The resistance R is

5
A potentiometer circuit has been set up for finding the internal resistance of a given cell. The main battery, used across the potentiometer wire, has an emf of 2.0 V and a negligible internal resistance. The potentiometer wire itself is 4 m long. When the resistance R, connected across the given cell, has values of (i) infinity, (ii) 9.5 $$\Omega $$
the balancing lengths on the potentiometer wire are found to be 3 m and 2.85 m, respectively. The value of internal resistance of the cell is
the balancing lengths on the potentiometer wire are found to be 3 m and 2.85 m, respectively. The value of internal resistance of the cell is
6
Two cities are 150 km apart. Electric power is sent from one city to another city through copper wires. The fall of potential per km is 8 volt and the average resistance per km is 0.5 $$\Omega $$. The power loss in the wire is
7
Two identical long conducting wires $$AOB$$ and $$COD$$ are placed at right angle to each other, with one above other such that $$O$$ is their common point for the two. The wires carry $$I$$1 and $$I$$2 currents, respectively. Point $$P$$ is lying at distance f from $$O$$ along a direction perpendicular to the plane containing the wires. The magnetic field at the point $$P$$ will be
8
In an ammeter 0.2% of main current passes through the galvanometer. If resistance of galvanometer is G, the resistance of ammeter will be
9
Following figures show the arrangement of bar magnets in different configurations. Each magnet has magnetic dipole moment $$\overrightarrow m .$$ Which configuration has highest net magnetic dipole moment ?


10
A thin semicircular conducting ring (PQR) of radius r is falling with its plane vertical in a horizontal magnetic field B, as shown in the figure.

The potential difference developed across the ring when its speed is $$v$$, is

The potential difference developed across the ring when its speed is $$v$$, is
11
A transformer having efficiency of 90% is working on 200 V and 3 kW power supply. If the current in the secondary coil is 6 A, the voltage across the secondary coil and the current in the primary coil respectively are
12
In a region, the potential is represented by V(x, y, z) = 6x $$-$$ 8xy $$-$$ 8y + 6yz, where $$V$$ is in volts and x, y, z are in metres. The electric force experienced by a charge of 2 coulomb situated at point (1, 1, 1) is
13
The angle of a prism is A. One of its refracting surfaces is silvered. Light rays falling at an angle of incidence 2 A on the first surface returns back through the same path after suffering reflection at the silvered surface.
The refractive index $$\mu $$, of the prism is
The refractive index $$\mu $$, of the prism is
14
A beam of light of $$\lambda = 600$$ nm from a distant source falls on a single slit 1 mm wide and the resulting diffraction pattern is observed on a screen 2 m away. The distance between first dark fringes on either side of the central bright fringe is
15
If the focal length of objective lens is increased then magnifying power of
16
In the Young's double slit experiment, the intensity of light at a point on the screen where the path difference $$\lambda $$ is K, ($$\lambda $$ being the wavelength of light used). The intensity at a point where the path difference is $$\lambda $$/4 will be
17
When the energy of the incident radiation is increased nby 20%, the kinetic energy of the photoelectrons emitted from a metal surface increased from 0.5 eV to 0.8 eV. The work function of the metal is
18
If the kinetic energy of the particle is increased to 16 times its previous value, the percentage change in the de Broglie wavelength of the particle is
19
Hydrogen atom in ground state is excited by a monochromatic radiation of $$\lambda $$ = 975 $$\mathop A\limits^ \circ $$. Number of spectral lines in the resulting spectrum emitted will be
20
The binding energy per nucleon of and nuclei are 5.60 MeV and 7.06 MeV respectively. In the nuclear reaction
$${}_3^7Li + {}_1^1H \to {}_2^4He + _2^4He + Q$$
the value of energy Q released is
$${}_3^7Li + {}_1^1H \to {}_2^4He + _2^4He + Q$$
the value of energy Q released is
21
The given graph represents V-I characteristic for a semiconductor device.

Which of the following statement is correct ?

Which of the following statement is correct ?
22
Copper of fixed volume V is drawn into wire of length $$l$$. When this wire is subjected to a constant force F, the extension produced in the wire is $$\Delta $$$$l$$. Which of the following graphs is a straight line ?
23
A projectile is fired from the surface of the earth with a velocity of 5 m s$$-$$1 and angle $$\theta $$
with the horizontal. Another projectile fired from another planet with a velocity of 3 m s$$-$$1 at the same angle follows a trajectory which is identical with the trajectory of the projectile fired from the earth. The value of the acceleration due to gravity on the planet is (in m s$$-$$2) is
(Given g = 9.8 m s$$-$$2)
(Given g = 9.8 m s$$-$$2)
24
A particle is moving such that is position coordinates (x, y) are (2 m, 3 m) at time t = 0, (6 m, 7 m)
at time t = 2 s and (13 m, 14 m) at time t = 5 s.
Average velocity vector $$\left( {{{\overrightarrow v }_{av}}} \right)$$ from t = 0 to t = 5 s is
at time t = 2 s and (13 m, 14 m) at time t = 5 s.
Average velocity vector $$\left( {{{\overrightarrow v }_{av}}} \right)$$ from t = 0 to t = 5 s is
25
A balloon with mass m is descending down with an acceleration a (where a < g). How much mass should be removed from it so that it starts moving up with an acceleration a ?
26
A system consists of three masses m1, m2 and m3 connected by a string passing over a pulley P. The mass m1 hangs freely and m2 and m3 are on a rough horizontal table (the coefficient of friction = $$\mu $$). The pulley is frictionless and of negligible mass. The downward acceleration of mass m1 is (Assume m1 = m2 = m3 = m)


27
The force F acting on a particle of mass m is indicated by the force-time graph shown below. The change in momentum of the particle over the time interval from zero to 8 s is :


28
A body of mass (4m) is lying in x-y plane at rest. It suddenly explodes into three pieces. Two pieces, each of mass (m) move perpendicular to each other with equal speeds (v). The total kinetic energy generated due to explosion is :
29
A solid cylinder of mass 50 kg and radius 0.5 m is free to rotate about the horizontal axis. A massless string is wound round the cylinder with one end attached to it and other hanging freely. Tension in the string required to produce an angular acceleration of 2 revolutions s$$-$$2 is
30
The ratio of the accelerations for a solid sphere (mass m and radius R) rolling down an incline of angle $$\theta $$ without slipping and slipping down the incline without rolling is
31
A black hole is an object whose gravitational field is so strong that even light cannot escape from it. To what approximate radius would earth (mass = 5.98 $$ \times $$ 1024 kg) have to be compressed to be a black hole?
32
Dependence of intensity of gravitational field (E) of earth with distance (r) from centre of earth is correctly represented by
33
The barrier potential of a p-n junction depends on
(1) type of semiconductor material
(2) amount of doping
(3) temperature
Which one of the following is correct ?
(1) type of semiconductor material
(2) amount of doping
(3) temperature
Which one of the following is correct ?
34
A certain number of spherical drops of a liquid of radius r coalesce to form a single drop of radius R and volume V. If T is the surface tension of the liquid, then
35
Steam at 100oC is passed into 20 g of water at 10oC. When water acquires a temperature of 80oC, the mass of water present will be
[Take specific heat of water = 1 cal g$$-$$1 oC$$-$$1 and latent heat of steam = 540 cal g$$-$$1]
[Take specific heat of water = 1 cal g$$-$$1 oC$$-$$1 and latent heat of steam = 540 cal g$$-$$1]
36
Certain quantity of water cools from 70oC to 60oC in the first 5 minutes and to 54oC in the next 5 minutes. The temperature of the surroundings is
37
A monatomic gas at a pressure P, having a volume V expands isothermally to a volume 2V and then adiabatically to a volume 16V. The final pressure of the gas is (Take $$\gamma $$ = 5/3)
38
A thermodynamic system undergoes cyclic process ABCDA as shown in figure. The work done by the system in the cycle is


39
The mean free path of molecules of a gas, (radius r) is inversely proportional to
40
The oscillation of a body on a smooth horizontal surface is represented by the equation,
X = A cos$$\left( {\omega t} \right)$$
where X = displacement at time t
$$\omega $$ = frequency of oscillation
Which one of the following graphs shows correctly the variation a with t?
Here a = acceleration at time t
T = time period
X = A cos$$\left( {\omega t} \right)$$
where X = displacement at time t
$$\omega $$ = frequency of oscillation
Which one of the following graphs shows correctly the variation a with t?
Here a = acceleration at time t
T = time period
41
If n1, n2 and n3 are the fundamental frequencies of three segments into which a string is divided, then the original fundamental frequency n of the string is given by
42
The number of possible natural oscillations of air column in a pipe closed at one end length 85 cm whose frequencies lie below 1250 Hz are (Velocity of sound = 340 m s$$-$$1)
43
If force (F), velocity (V) and time (T) are taken as fundamental units, then dimensions of mass are